Question: Question 6 Determine the oxidation number of manganese (Mn) in each of the following compounds. [Select] [Select] +3 -3 +2 (1) Mn03 (2) MnO
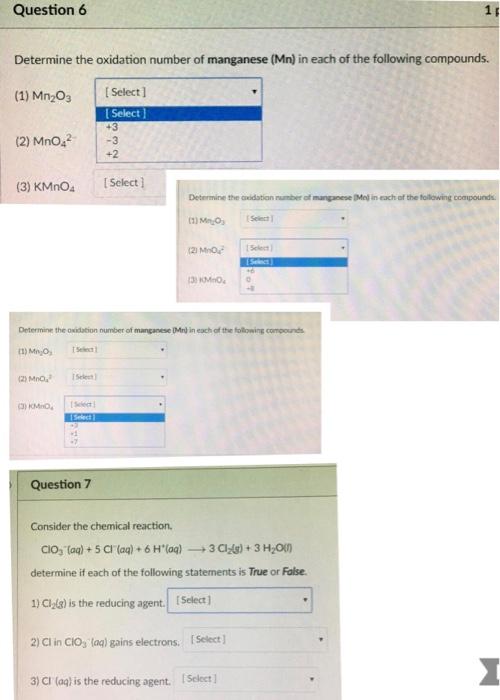
Question 6 Determine the oxidation number of manganese (Mn) in each of the following compounds. [Select] [Select] +3 -3 +2 (1) Mn03 (2) MnO (3) KMnO4 (3) KMnO [Select] [Select] [Select c Question 7 [Select] Determine the oxidation number of manganese (Mel in each of the following compounds. (1) MO (2) MO (3) KMnO Determine the oxidation number of manganese (Mr) in each f the following compounds (1) MnO, [Select] (2) MnO [Select 2) Cl in CIO, (aq) gains electrons. [Select] [Select Select 3) CI (aq) is the reducing agent. [Select] O 4 Consider the chemical reaction. CIO (aq) + 5 CI (aq) + 6 H(aq) 3 Cl(g) + 3 HO(l) determine if each of the following statements is True or False. 1) Cl(g) is the reducing agent. [Select] 1
Step by Step Solution
3.33 Rating (147 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


