Question: Select the TRUE statements from the list below. Select all that apply. Li has more metallic character than F. Fand Na+have the same ionic radius.
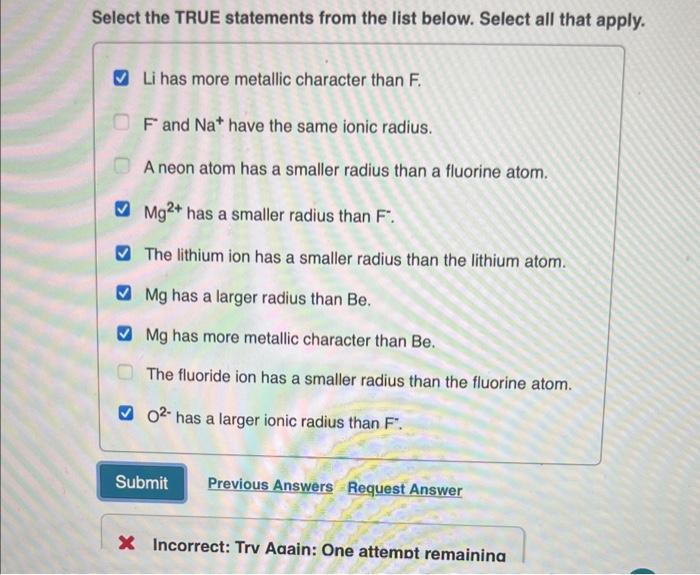
Select the TRUE statements from the list below. Select all that apply. Li has more metallic character than F. Fand Na+have the same ionic radius. A neon atom has a smaller radius than a fluorine atom. Mg2+ has a smaller radius than F. The lithium ion has a smaller radius than the lithium atom. Mg has a larger radius than Be. Mg has more metallic character than Be. The fluoride ion has a smaller radius than the fluorine atom. O2 has a larger ionic radius than F. Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


