Question: Selenium (IV) in natural waters can be determined by complexing with ammonium pyrrolidine dithiocarbamate and extracting into CHC13. This step serves to concentrate the Se(IV)
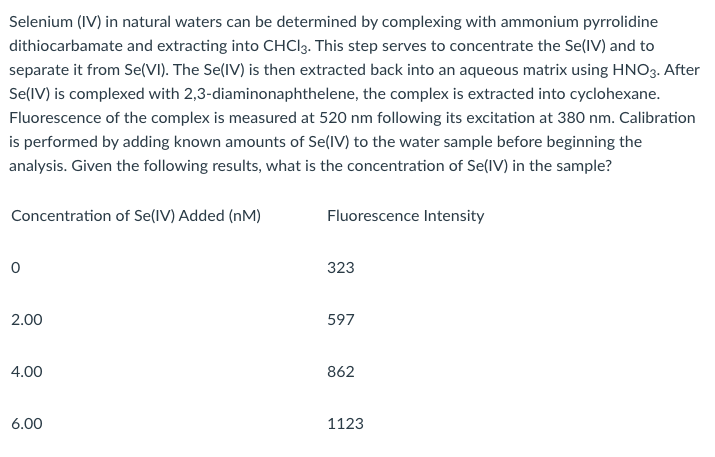
Selenium (IV) in natural waters can be determined by complexing with ammonium pyrrolidine dithiocarbamate and extracting into CHC13. This step serves to concentrate the Se(IV) and to separate it from Se(VI). The Se(IV) is then extracted back into an aqueous matrix using HNO3. After Se(IV) is complexed with 2,3-diaminonaphthelene, the complex is extracted into cyclohexane. Fluorescence of the complex is measured at 520 nm following its excitation at 380 nm. Calibration is performed by adding known amounts of Se(IV) to the water sample before beginning the analysis. Given the following results, what is the concentration of Se(IV) in the sample? Concentration of Se(IV) Added (nM) Fluorescence Intensity 0 323 2.00 597 4.00 862 6.00 1123
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


