Question: Self test 4a Additional Problems in Chapter 5: 4.40. Classify each compound as a strong 41,43,45,48,56. electrolyte or nonelectrolyte. 5.55. Write balanced complete ionic and
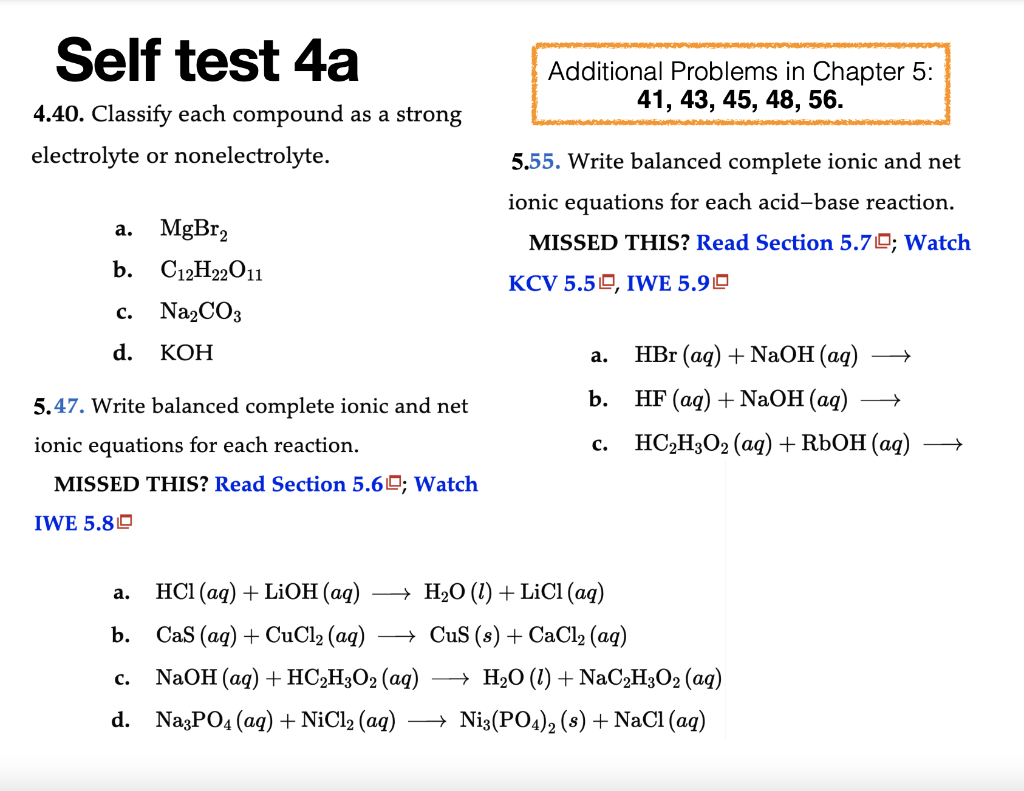
Self test 4a Additional Problems in Chapter 5: 4.40. Classify each compound as a strong 41,43,45,48,56. electrolyte or nonelectrolyte. 5.55. Write balanced complete ionic and net ionic equations for each acid-base reaction. a. MgBr2 MISSED THIS? Read Section 5.7 ; Watch b. C12H22O11 KCV 5.5, IWE 5.9! c. Na2CO3 d. KOH a. HBr(aq)+NaOH(aq) 5.47. Write balanced complete ionic and net b. HF(aq)+NaOH(aq) ionic equations for each reaction. c. HC2H3O2(aq)+RbOH(aq) MISSED THIS? Read Section 5.6 ; Watch IWE 5.8 a. HCl(aq)+LiOH(aq)H2O(l)+LiCl(aq) b. CaS(aq)+CuCl2(aq)CuS(s)+CaCl2(aq) c. NaOH(aq)+HC2H3O2(aq)H2O(l)+NaC2H3O2(aq) d. Na3PO4(aq)+NiCl2(aq)Ni3(PO4)2(s)+NaCl(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


