Question: Predict what will happen to the conductivity of a solution when HCI is titrated with NaOH. Draw a predicted curve and explain why you
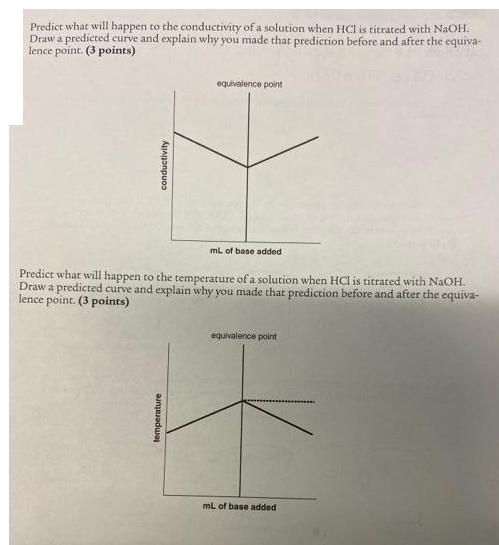
Predict what will happen to the conductivity of a solution when HCI is titrated with NaOH. Draw a predicted curve and explain why you made that prediction before and after the equiva- lence point. (3 points) conductivity equivalence point mL of base added Predict what will happen to the temperature of a solution when HCl is titrated with NaOH. Draw a predicted curve and explain why you made that prediction before and after the equiva- lence point. (3 points) temperature equivalence point mL of base added
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
1 The titration of HCl hydrochloric acid with NaOH sodium hydroxide is a classic example of an acidbase titration In this titration the conductivity of the solution will change as you add the base NaO... View full answer

Get step-by-step solutions from verified subject matter experts


