Question: separation process = D14.* We are operating a staged distillation column at total reflux to determine the Murphree efficiency. Pressure is 101.3 kPa. We are
separation process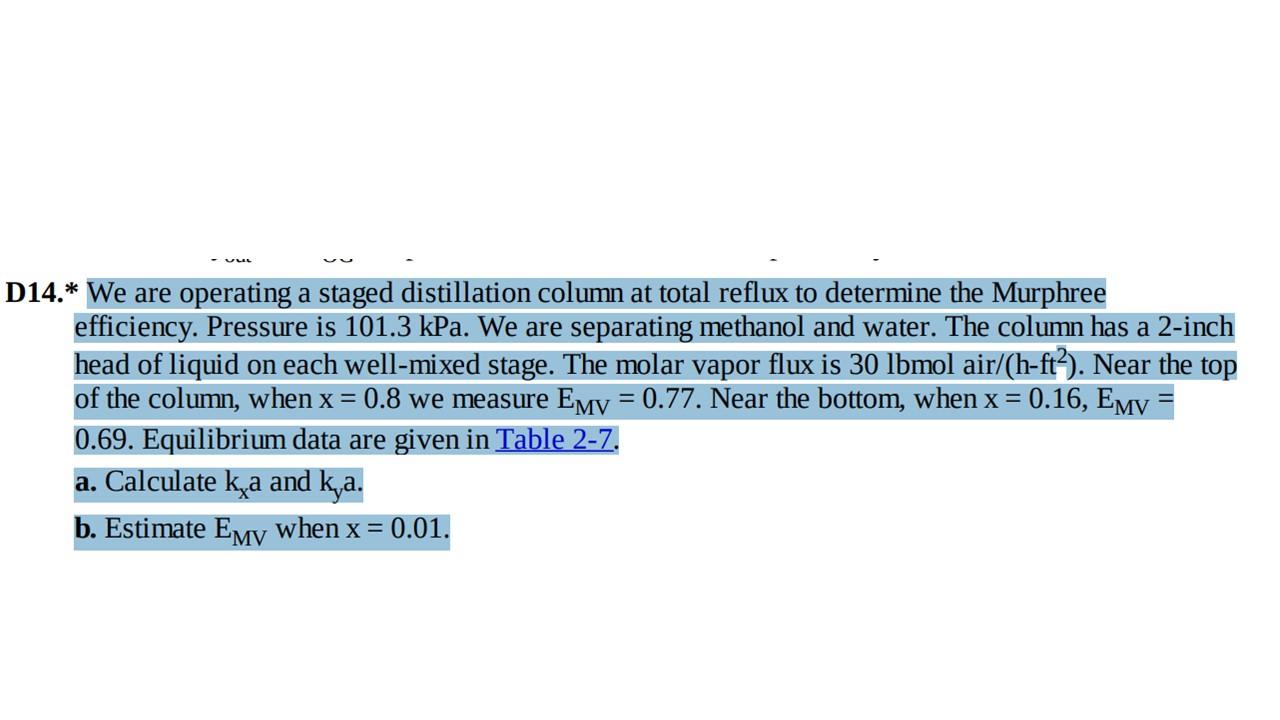
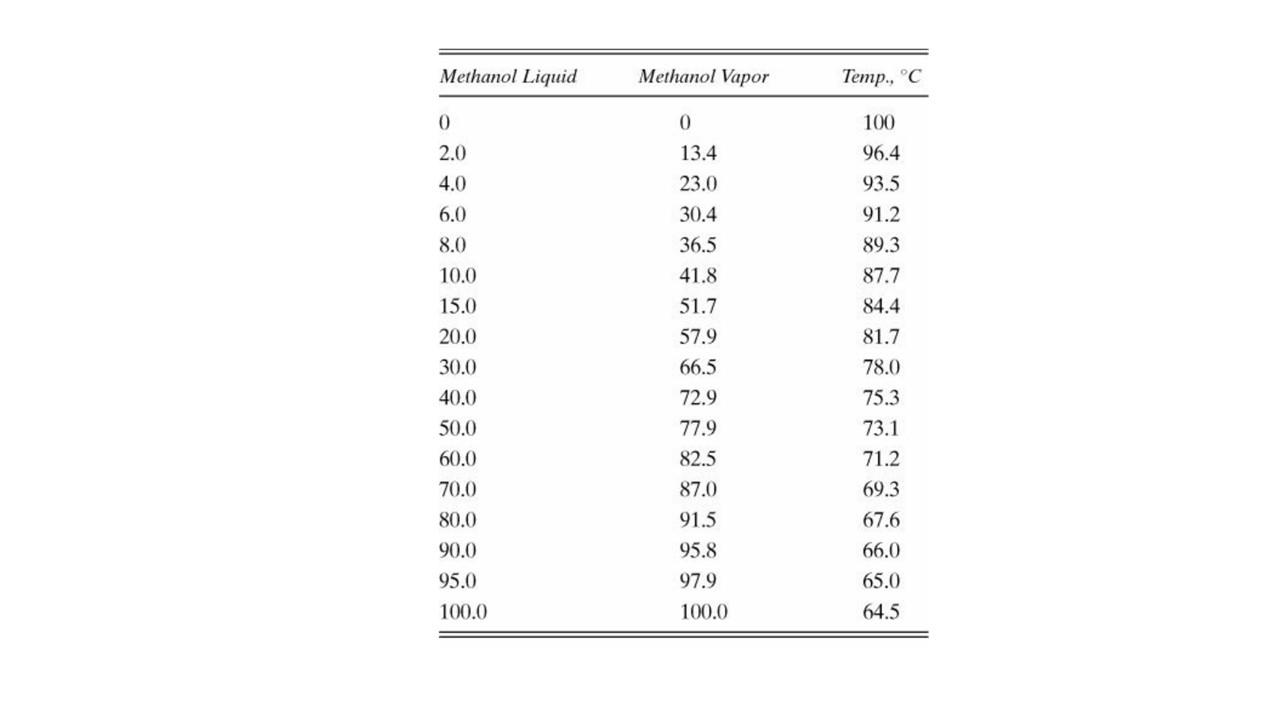
= D14.* We are operating a staged distillation column at total reflux to determine the Murphree efficiency. Pressure is 101.3 kPa. We are separating methanol and water. The column has a 2-inch head of liquid on each well-mixed stage. The molar vapor flux is 30 lbmol air/(h-ft?). Near the top of the column, when x = 0.8 we measure Emv = 0.77. Near the bottom, when x = 0.16, EMV 0.69. Equilibrium data are given in Table 2-7. a. Calculate ka and ka. b. Estimate Emy when x = 0.01. = Methanol Liquid Methanol Vapor Temp., C 0 0 13.4 23.0 30.4 36.5 41.8 51.7 2.0 4.0 6.0 8.0 10.0 15.0 20.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 95.0 100.0 57.9 66.5 72.9 77.9 82.5 87.0 91.5 95.8 97.9 100.0 100 96.4 93.5 91.2 89.3 87.7 84.4 81.7 78.0 75.3 73.1 71.2 69.3 67.6 66.0 65.0 64.5 = D14.* We are operating a staged distillation column at total reflux to determine the Murphree efficiency. Pressure is 101.3 kPa. We are separating methanol and water. The column has a 2-inch head of liquid on each well-mixed stage. The molar vapor flux is 30 lbmol air/(h-ft?). Near the top of the column, when x = 0.8 we measure Emv = 0.77. Near the bottom, when x = 0.16, EMV 0.69. Equilibrium data are given in Table 2-7. a. Calculate ka and ka. b. Estimate Emy when x = 0.01. = Methanol Liquid Methanol Vapor Temp., C 0 0 13.4 23.0 30.4 36.5 41.8 51.7 2.0 4.0 6.0 8.0 10.0 15.0 20.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 95.0 100.0 57.9 66.5 72.9 77.9 82.5 87.0 91.5 95.8 97.9 100.0 100 96.4 93.5 91.2 89.3 87.7 84.4 81.7 78.0 75.3 73.1 71.2 69.3 67.6 66.0 65.0 64.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


