Question: Short Answer. Answer the questions in the space provided. 14. Let's consider the structure and combustion of oxalic acid (C2H2O4). a) (3 pts.) Complete the
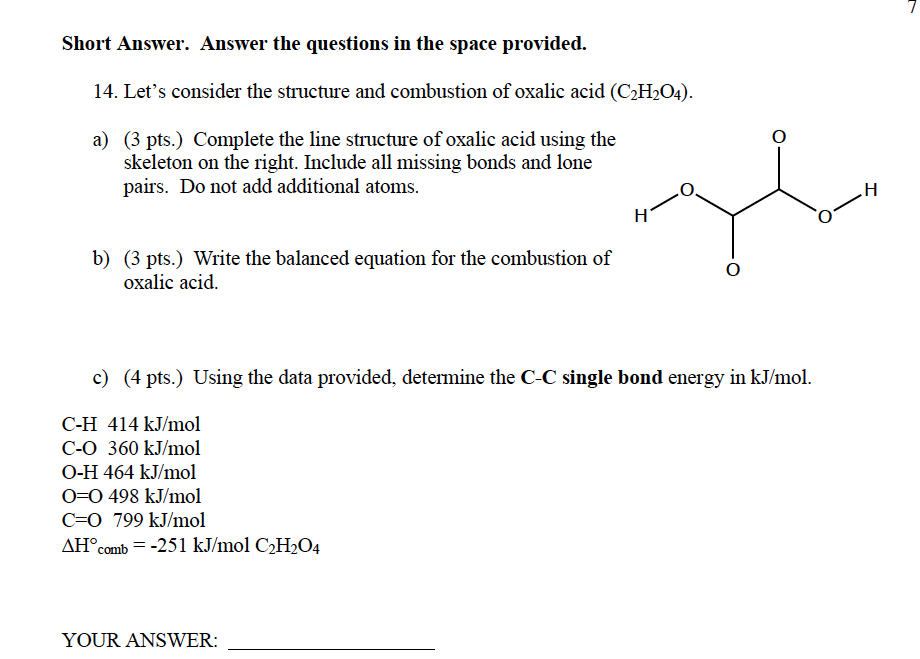
Short Answer. Answer the questions in the space provided. 14. Let's consider the structure and combustion of oxalic acid (C2H2O4). a) (3 pts.) Complete the line structure of oxalic acid using the skeleton on the right. Include all missing bonds and lone pairs. Do not add additional atoms. b) (3 pts.) Write the balanced equation for the combustion of oxalic acid. c) (4 pts.) Using the data provided, determine the C-C single bond energy in kJ/mol. CH414kJ/mol CO360kJ/mol OH464kJ/mol O=O498kJ/mol C=O799kJ/mol Hcomb=251kJ/molC2H2O4 YOUR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


