Question: SHOW ALL CALCULATIONS AND CARRY THE UNITS through each step! Our laboratory is interested in factors affecting the activity of phenylalanine ammonia lyase an enzyme
SHOW ALL CALCULATIONS AND CARRY THE UNITS through each step!
Our laboratory is interested in factors affecting the activity of phenylalanine ammonia lyase an enzyme that catalyzes the conversion of phenylalanine to trans cinnamic acid (TCA). We therefore needed an assay to quantify TCA.
We developed an HPLC method with a mobile phase of 25 mM Phosphate buffer (pH 6.5): Acetonitrile in a 92:8 ratio. The mobile phase was pumped at a flow rate of 1.5 mL/min and the eluent was separated on a Zorbax Eclipse Plus C18 4.6 x 150 mm, 3.5 m column. TCA was detected using UV absorbance at a wavelength of 270 nm. The retention time of TCA in this system was 7.4 mins.
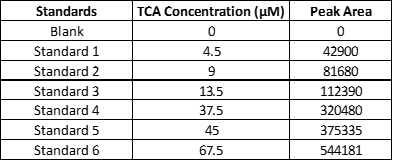
Listed above are absorbance (expressed as peak area) versus concentration data for a set of TCA standards using our system.
1. Plot the data on a linear-linear scale with peak area (absorbance) on the y-axis and concentration on the x-axis. Using linear regression, calculate the extinction coefficient of trans cinnamic acid. Please include its units.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


