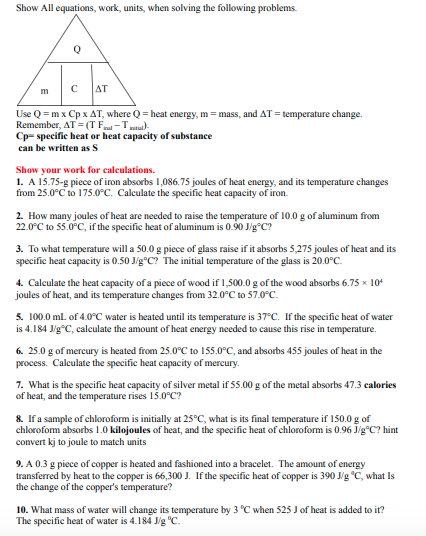
Question: Show All equations. work. units. when solving the following problems. Use Q = m x C p x T , where Q = heat energy,
Show All equations. work. units. when solving the following problems.
Use where heat energy, mass, and temperature change. Remember,
specific heat or heat capacity of substance
can be written as
Show your work for calculations.
A piece of iron absorbs joules of heat energy, and its temperature changes from to Calculate the specific heat capacity of iron.
How many joules of heat are needed to raise the temperature of of aluminum from to if the specific heat of aluminum is
To what temperature will a piece of glass raise if it absorbs joules of heat and its specific heat capacity is The initial temperature of the glass is
Calculate the heat capacity of a piece of wood if of the wood absorbs joules of heat, and its temperature changes from to
of water is heated until its temperature is If the specific heat of water is calculate the amount of heat energy needed to cause this rise in temperature.
of mercury is beated from to and absorbs joules of heat in the process. Calculate the specific heat capacity of mercury.
What is the specific heat capacity of silver metal if of the metal absorbs calories of heat, and the temperature rises
If a sample of chloroform is initially at what is its final temperature if of chloroform absorbs kilojoules of heat, and the specific heat of chloroform is hint convert kj to joule to match units
A piece of copper is heated and fashioned into a bracelet. The amount of energy transferred by heat to the copper is If the specific heat of copper is what Is the change of the copper's temperature?
What mass of water will change its temperature by when of heat is added to it The specific heat of water is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


