Question: Show all work plz 5. For a particular binary mixture at constant T and P, the molar enthalpy of the mixture can be represented of

Show all work plz
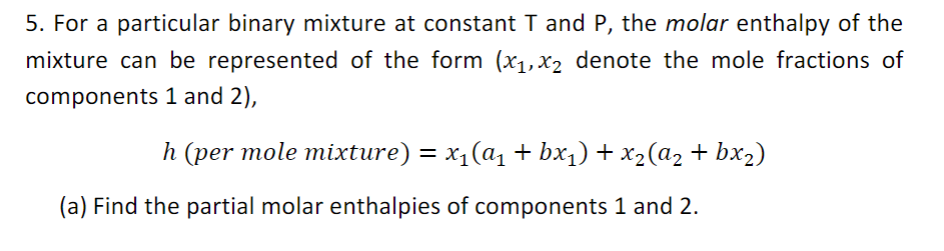
5. For a particular binary mixture at constant T and P, the molar enthalpy of the mixture can be represented of the form (x1,x2 denote the mole fractions of components 1 and 2), h (per mole mixture) = x1(a1 + bx1) + x2(a2 + bx2) = (a) Find the partial molar enthalpies of components 1 and 2. For the parts below, consider a mixing process in which stream 1 contains one mole of component 1 and stream 2 contains one mole of a 50-50 mixture (by mole) of components 1 and 2. The specific heats of the components 1 and 2 are denoted (+) and (2) respectively. The initial temperatures of streams 1 and 2 are the same and denoted as Ti. (b) If the mixing process happens isothermally, what is the heat released? (c) If the mixing process happens adiabatically, what is the final temperature of the mixture
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


