Question: Show Attempt History Current Attempt in Progress As shown in the figure below, a gas contained within a piston - cylinder assembly, initially at a
Show Attempt History
Current Attempt in Progress
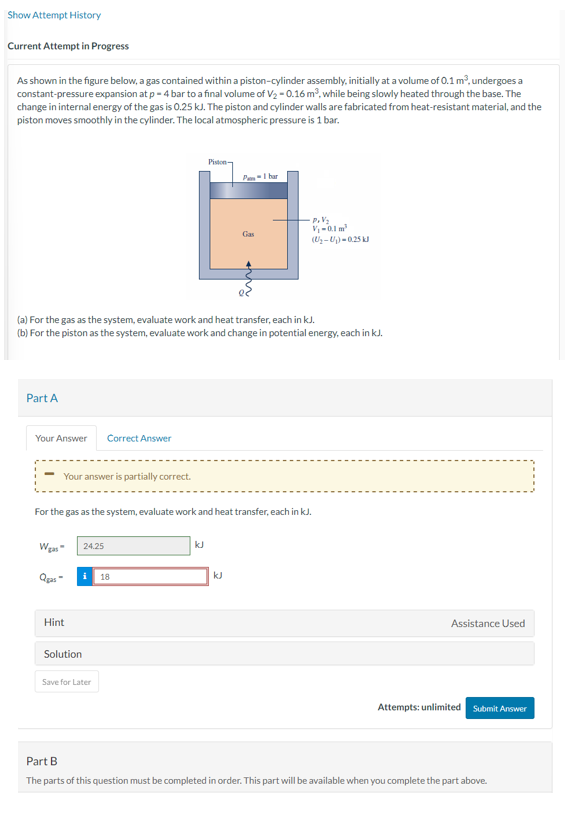
As shown in the figure below, a gas contained within a pistoncylinder assembly, initially at a volume of undergoes a
constantpressure expansion at bar to a final volume of while being slowly heated through the base. The
change in internal energy of the gas is kJ The piston and cylinder walls are fabricated from heatresistant material, and the
piston moves smoothly in the cylinder. The local atmospheric pressure is bar.
a For the gas as the system, evaluate work and heat transfer, each in kJ
b For the piston as the system, evaluate work and change in potential energy, each in kJ
Part A
Correct Answer
Your answer is partially correct.
For the gas as the system, evaluate work and heat transfer, each in kJ
Hint
Solution
Part B
The parts of this question must be completed in order. This part will be available when you complete the part above.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


