Question: Show Attempt History Current Attempt in Progress * Make sure to distinguish kJ/mol and J/mol. You are writing energy balances for a compound for which
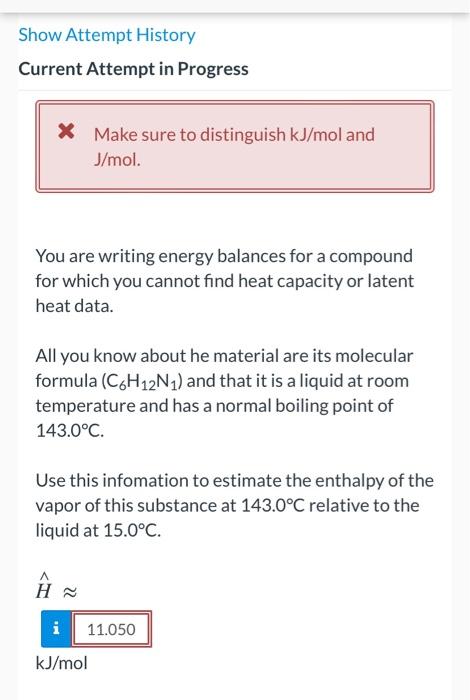
Show Attempt History Current Attempt in Progress * Make sure to distinguish kJ/mol and J/mol. You are writing energy balances for a compound for which you cannot find heat capacity or latent heat data. All you know about he material are its molecular formula (C6H12N1) and that it is a liquid at room temperature and has a normal boiling point of 143.0C. Use this infomation to estimate the enthalpy of the vapor of this substance at 143.0C relative to the liquid at 15.0C. H i 11.050 kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


