Question: show complete and detailed solution 4. Experimental data shows the water-alcohol proportion at 60C Mole fraction of 0.00 0.05 0.27 0.44 0.69 0.83 1.00 water
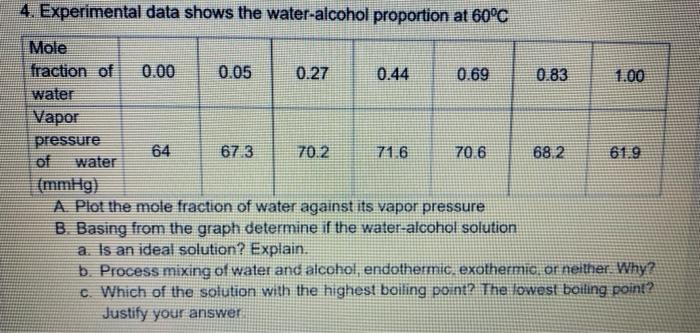
4. Experimental data shows the water-alcohol proportion at 60C Mole fraction of 0.00 0.05 0.27 0.44 0.69 0.83 1.00 water Vapor pressure 64 67.3 70.2 71 6 70.6 68.2 61.9 of water (mmHg) A Plot the mole fraction of water against its vapor pressure B. Basing from the graph determine if the water-alcohol solution a. Is an ideal solution? Explain. b. Process mixing of water and alcohol, endothermic. exothermio, or neither. Why? c. Which of the solution with the highest boiling point? The lowest boiling point? Justify your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


