Question: SHOW COMPLETE SOLUTION AND WRITE THE ANSWERS IN 4 DECIMAL PLACES Equilibria in Hydrates The equilibrium constants for the dissociation of hydrates are given simply
SHOW COMPLETE SOLUTION AND WRITE THE ANSWERS IN 4 DECIMAL PLACES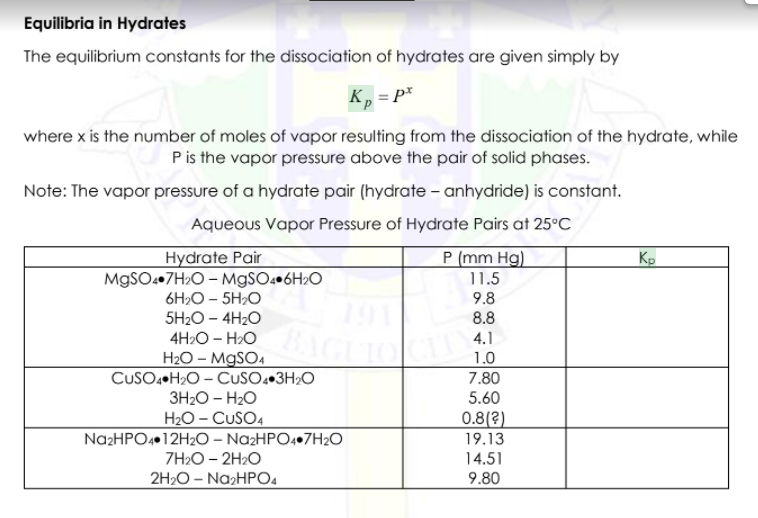

Equilibria in Hydrates The equilibrium constants for the dissociation of hydrates are given simply by K, =P where x is the number of moles of vapor resulting from the dissociation of the hydrate, while Pis the vapor pressure above the pair of solid phases. Note: The vapor pressure of a hydrate pair (hydrate - anhydride) is constant. Aqueous Vapor Pressure of Hydrate Pairs at 25C Hydrate Pair P (mm Hg) MgSO4.7H2O - MgSO4 6H2O 11.5 6H20-5H20 9.8 5H20 - 4H20 8.8 4H20 - H2O 4.1 H2O - MgSO4 1.0 CuSO4+H2O- CUSO43H2O 7.80 3H20 - H2O 5.60 H2O- CUSO4 0.8(2) Na2HPO4 12H20 - Na2HPO4.7H2O 19.13 7H20-2H2O 14.51 2H2O- Na2HPO4 9.80 PROBLEM 4: Given the reaction: CuSO4 3H2O.is CuSO4 2Hz0is) + H2O) the AH = 19800 and AGO = 9000 at 25C. Calculate the partial pressure of water vapor at 700K The AH is independent of temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


