Question: Show full process ( 2 5 points ) Consider m = 1 K g of nitrogen ( N 2 ) gas being compressed within the
Show full process
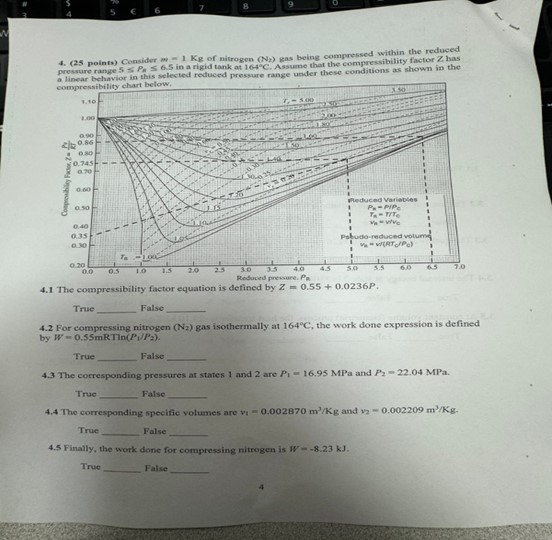
points Consider of nitrogen N gas being compressed within the reduced pressure range in a rigid tank at Assume that the compressibility factor has presed reduced pressure range under these conditions as shown in the
The compressibility factor equation is defined by
True False
For compressing nitrogen gas isothermally at the work done expression is defined by
True False
The corresponding pressures at states and are MPa and MPa.
True False
The corresponding specific volumes are and True False
Finally, the work done for compressing nitrogen is False
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


