Question: show how to get answer 3.6% wt/wt A 0.350g sample of an iron bearing ore was analysed for iron by AAS. The ore was digested
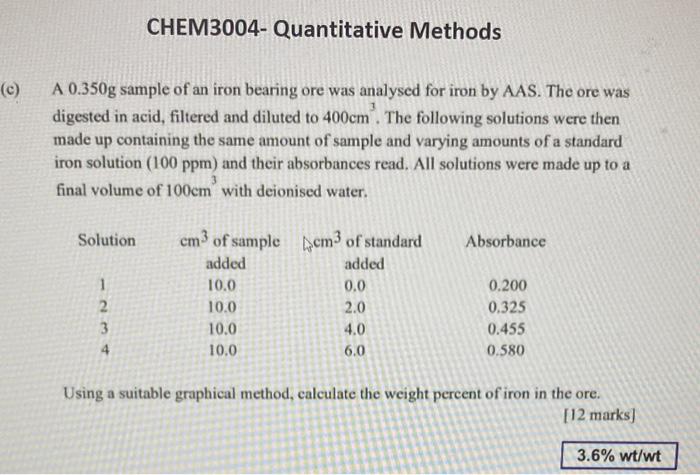
A 0.350g sample of an iron bearing ore was analysed for iron by AAS. The ore was digested in acid, filtered and diluted to 400cm3. The following solutions were then made up containing the same amount of sample and varying amounts of a standard iron solution ( 100ppm) and their absorbances read. All solutions were made up to a final volume of 100cm3 with deionised water. Using a suitable graphical method, calculate the weight percent of iron in the ore. [12 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


