Question: Show the step by step derivation to come up with the same result. All trimolecular reactions found so far are of the form of Eq.
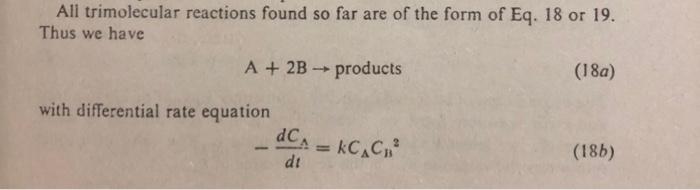
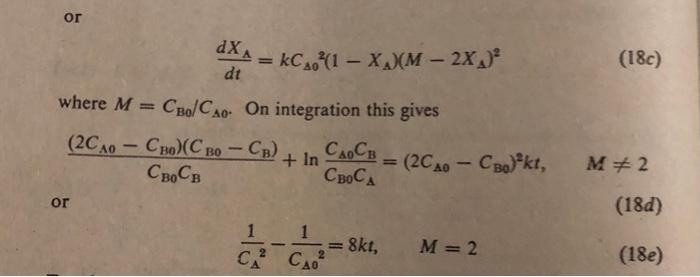

All trimolecular reactions found so far are of the form of Eq. 18 or 19. Thus we have A+2Bproducts with differential rate equation dtdCA=kCCB2 dtdXA=kC02(1XA)(M2XA)2 where M=CB0/CA0. On integration this gives CB0CB(2CA0CB0)(CB0CB)+lnCB0CACA0CB=(2CA0CB0)2kt,M=2 or CA21CA021=8kt,M=2 Derive the integrated form of the rate expression in terms of Xa of the trimolecular reaction A+2Bproducts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


