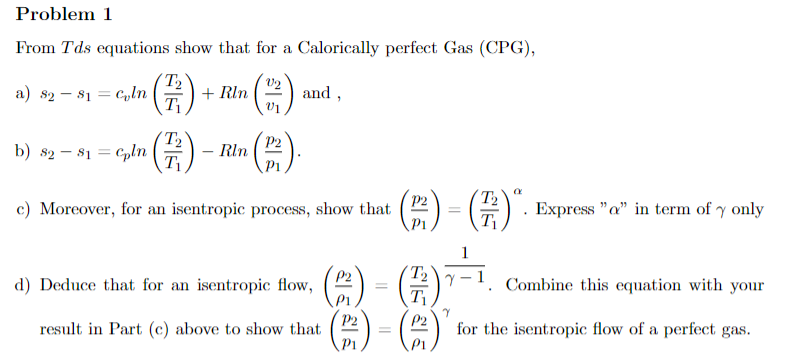
Question: ( Show work and circle / box final answers ) Problem 1 From T d s equations show that for a Calorically perfect Gas (
Show work and circlebox final answersProblem
From equations show that for a Calorically perfect Gas CPG
a and
b
c Moreover, for an isentropic process, show that Express in term of only
d Deduce that for an isentropic flow, Combine this equation with your
result in Part c above to show that for the isentropic flow of a perfect gas.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


