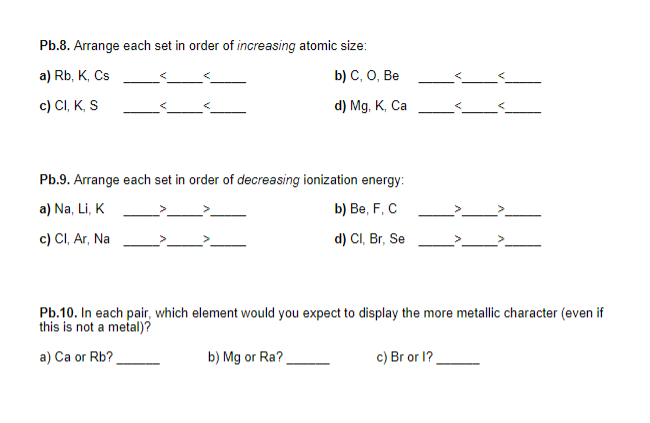
Question: show work for all 3 problems please and thank you Pb.8. Arrange each set in order of increasing atomic size: a) Rb, K, Cs b)
show work for all 3 problems please and thank you
Pb.8. Arrange each set in order of increasing atomic size: a) Rb, K, Cs b) C, O, Be c) CI, KS d) Mg, K, Ca Pb.9. Arrange each set in order of decreasing ionization energy: a) Na, Li, K b) Be, F, C c) CI, Ar, Na d) CI, Br, Se Pb.10. In each pair, which element would you expect to display the more metallic character (even if this is not a metal)? a) Ca or Rb? b) Mg or Ra? c) Bror 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


