Question: Show work PLEASE 2. [20] Nitrate is an alternative electron acceptor to oxygen for the oxidation of acetate. 5CH3COO + 8NO3+ + 3H+ = 10HCO3+
![Show work PLEASE 2. [20] Nitrate is an alternative electron acceptor](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d73d3c290_06066f8d73cba284.jpg)
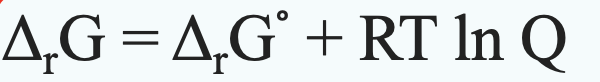
Show work PLEASE
2. [20] Nitrate is an alternative electron acceptor to oxygen for the oxidation of acetate. 5CH3COO + 8NO3+ + 3H+ = 10HCO3+ + 4N2(g) + 4H2O a) For a system at pH 7 in equilibrium with 0.81 atm N2(g) and 1 g/L acetate, 1 mM HCO3 , and 1 mM NO3 , what is the Gibbs free energy of the reaction? Give your answer in kJ per mole of acetate oxidized. The standard molar Gibbs free energy of formation of H+ and N2(g) are 0. b) How does this answer compare to that calculated in class for the oxidation of acetate using O2? Which metabolism (oxygen use versus nitrate reduction) is more energetically favorable? 4,G=A,G + RT In
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


