Question: show work Solve the problem in your notes. SHOW ALL WORK! Type your numeric answer below. Find the AH for the reaction below, given the
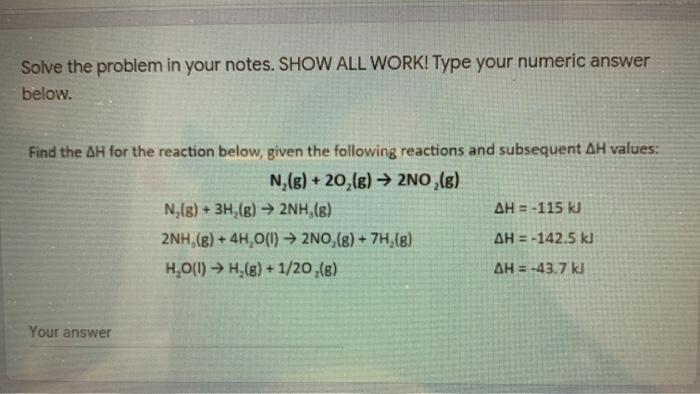
Solve the problem in your notes. SHOW ALL WORK! Type your numeric answer below. Find the AH for the reaction below, given the following reactions and subsequent AH values: N,(g) + 20,(g) 2NO,(g) N (s) + 3H (6) 2NH,(8) AH = -115 kJ 2NH (8) + 4H,0(1) 2NO,(8) + 7H (8) AH = -142.5 kl H.O(1) H,(8)+1/20 (8) AH = -43.7 kJ Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


