Question: Shown are four Lewis structures for ozone (0,). Match each structure (on the left) with the correct response (on the right). A. 10-0-01 B.
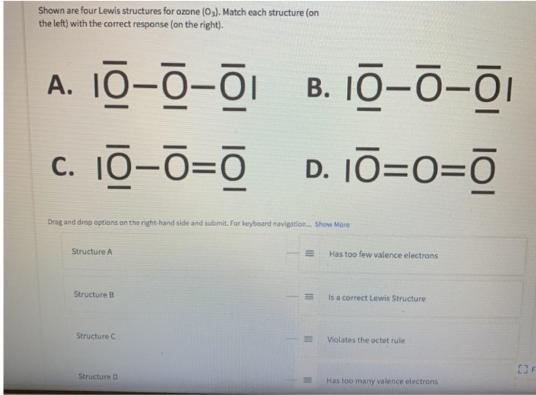
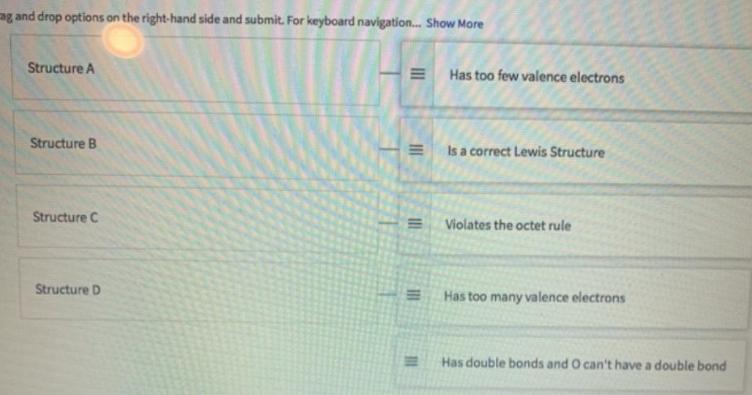
Shown are four Lewis structures for ozone (0,). Match each structure (on the left) with the correct response (on the right). A. 10-0-01 B. 10--i c. 10-0=0 D. 10=0=O Drag and dine optians an the rght hand side and mit. For beybeard navigation tho Mare Structure A Has too few valence electrans Structure is a correct Lewis Structure Structure C Violates the uctet rule Structurm D Has too many valence electrons g and drop options on the right-hand side and submit. For keyboard navigation. Show More Structure A Has too few valence electrons Structure B Is a correct Lewis Structure Structure C Violates the octet rule Structure D Has too many valence electrons Has double bonds and O can't have a double bond II II
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
In O 3 total number of valence electrons 63 18 es In structure A total num... View full answer

Get step-by-step solutions from verified subject matter experts


