Question: Show your work. I will upvote if you solve quickly... 19) Consider the following equilibrium systems: A(g)B(g)Kc=2 and B(g)C(g)Kc=0.01 What is the value of Kc
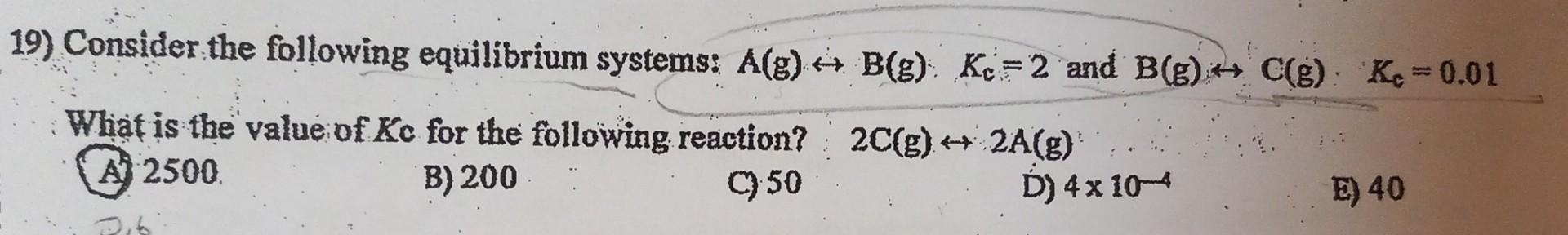
Show your work. I will upvote if you solve quickly...
19) Consider the following equilibrium systems: A(g)B(g)Kc=2 and B(g)C(g)Kc=0.01 What is the value of Kc for the following reaction? 2C(g)2A(g) A 2500 B) 200 C) 50 D) 4104 E) 40
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


