Question: Show your work. No work shown means no points. Make sure to use units and the correct number of sig figs. Consider a series of
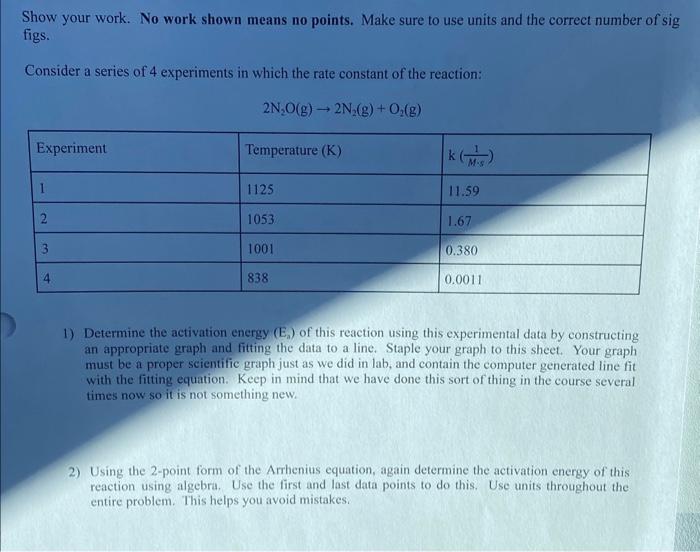
Show your work. No work shown means no points. Make sure to use units and the correct number of sig figs. Consider a series of 4 experiments in which the rate constant of the reaction: 2N2O(g)2N2(g)+O2(g) 1) Determine the activation energy (Ea) of this reaction using this experimental data by constructing an appropriate graph and fitting the data to a line. Staple your graph to this sheet. Your graph must be a proper scientific graph just as we did in lab, and contain the computer generated line fit with the fitting equation. Keep in mind that we have done this sort of thing in the course several times now so it is not something new. 2) Using the 2-point form of the Arrhenius equation, again determine the activation energy of this reaction using algebra. Use the first and last data points to do this. Use units throughout the entire problem. This helps you avoid mistakes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


