Question: Shown below is a flow diagram created by Peter, Erron and Abigail for the manufacture of nitric acid by the ammonia-oxidation process. The diagram
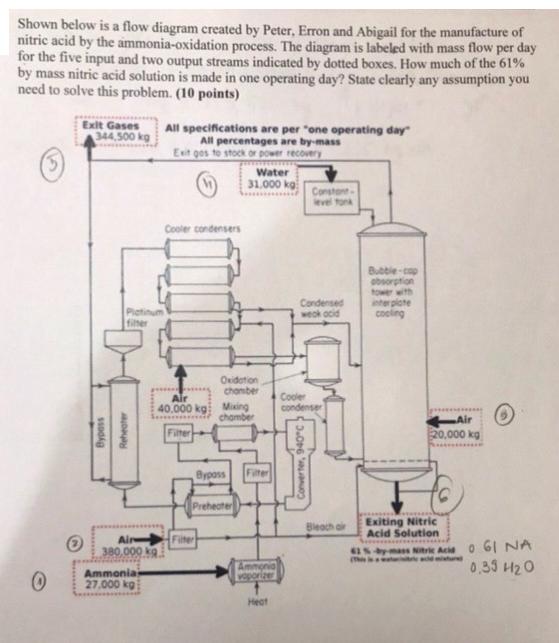
Shown below is a flow diagram created by Peter, Erron and Abigail for the manufacture of nitric acid by the ammonia-oxidation process. The diagram is labeled with mass flow per day for the five input and two output streams indicated by dotted boxes. How much of the 61% by mass nitric acid solution is made in one operating day? State clearly any assumption you need to solve this problem. (10 points) 0 Exit Gases 344,500 kg Platinum filter Bypass Reheater 380,000 kg Ammonia 27,000 kg All specifications are per "one operating day" All percentages are by-mass Exit gos to stock or power recovery Cooler condensers Air 40.000 kg: Filter Filter Oxidation chamber Water 31.000 kg Mixing chamber Bypass Preheater Filter Ammonia vaporizer Heat Constant- level tonk Condensed weak acid Cooler condenser Converter, 940C) Bleach air Bubble-cop absorption tower with (The interplate cooling Air 20,000 kg PC Exiting Nitric Acid Solution 61%-by-mass Nitric Acid 061 NA 0.33 420
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
There are 5 input streams and 2 output streams Overall mass is conserved Mass in mass ou... View full answer

Get step-by-step solutions from verified subject matter experts


