Question: Shown below is the infrared spectrum for a molecule with the molecular formula of C9H10O2. An infrared table is provided on the last page of
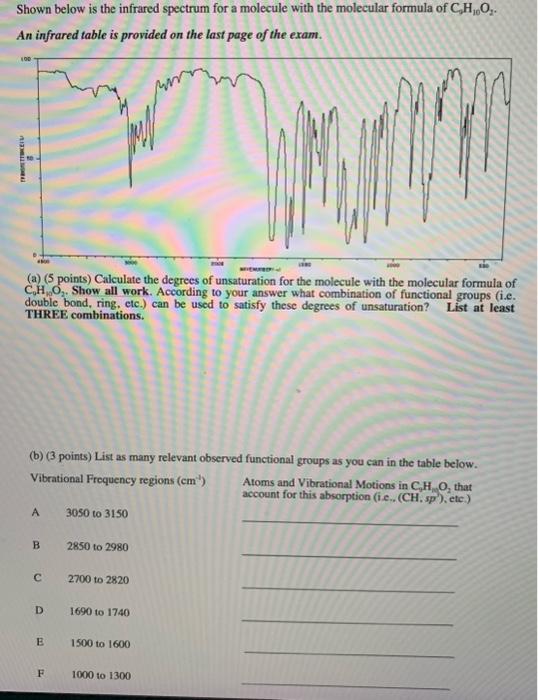
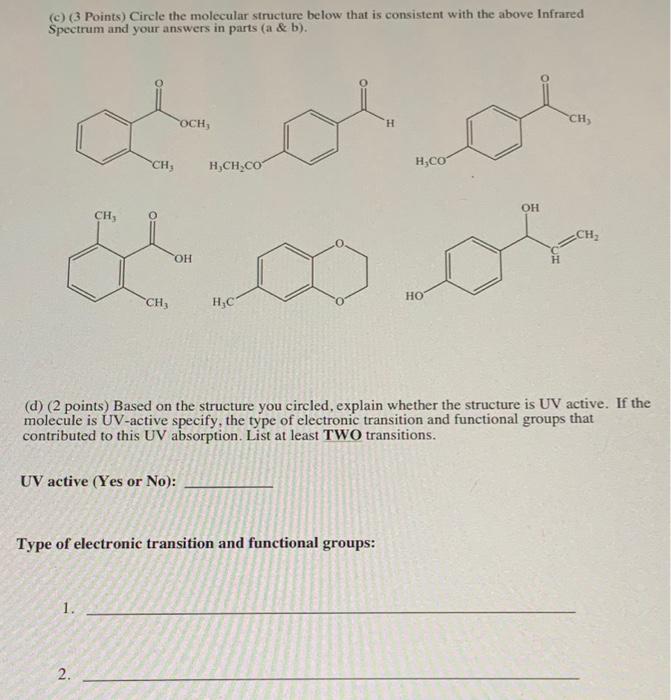
Shown below is the infrared spectrum for a molecule with the molecular formula of C9H10O2. An infrared table is provided on the last page of the exam. (a) (5 points) Calculate the degrees of unsaturation for the molecule with the molecular formula of C1H2O2. Show all work. According to your answer what combination of functional groups (i.e. double bond, ring, etc.) can be used to satisfy these degrees of unsaturation? List at least THREE combinations. (b) (3 points) List as many relevant observed functional groups as you can in the table below. Vibrational Frequency regions (cm1) Atoms and Vibrational Motions in C4H2O2, that account for this absorption (i.e., (CH,sp ), ete.) A 3050t03150 B 2850 to 2980 C 2700 to 2820 D 1690 to 1740 B 1500 to 1600 F 1000 to 1300 (c) (3 Points) Circle the molecular structure below that is consistent with the above Infrared Spectrum and your answers in parts (a \& b). (d) (2 points) Based on the structure you circled, explain whether the structure is UV active. If the molecule is UV-active specify, the type of electronic transition and functional groups that contributed to this UV absorption. List at least TWO transitions. UV active (Yes or No)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


