Question: Shown in the figure below is a classical model (i.e. before we knew about quantum mechanics) of an atom. Here an electron (m = 9.109e-31
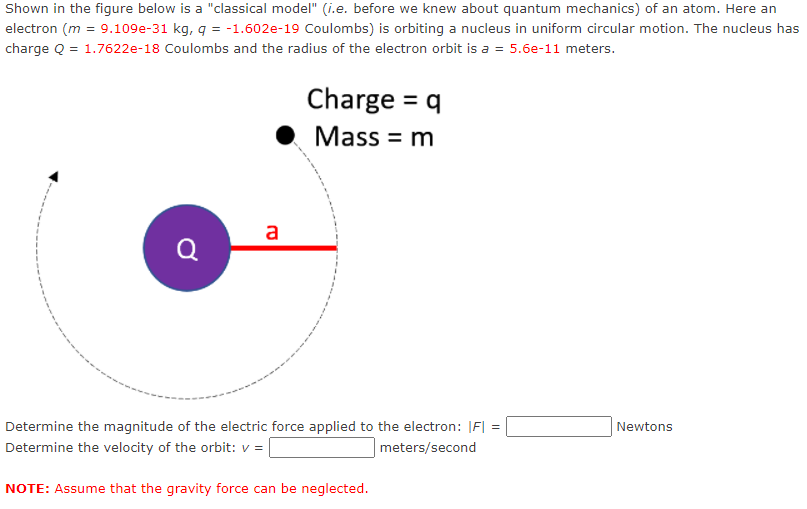
Shown in the figure below is a "classical model" (i.e. before we knew about quantum mechanics) of an atom. Here an electron (m = 9.109e-31 kg, q = -1.602e-19 Coulombs) is orbiting a nucleus in uniform circular motion. The nucleus has charge Q = 1.7622e-18 Coulombs and the radius of the electron orbit is a = 5.6e-11 meters. Charge = q Mass = m a Q Determine the magnitude of the electric force applied to the electron: IF| = Newtons Determine the velocity of the orbit: v = meters/second NOTE: Assume that the gravity force can be neglected
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


