Question: Silver bromide is produced in large quantities for use in making black-and-white photographs by combining silver nitrate with potassium bromide: AgNO3(aq)+KBr(aq)AgBr(s)+KNO3(aq) Consider a reaction between
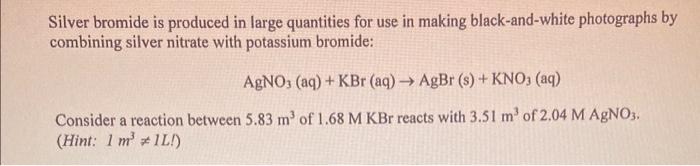

Silver bromide is produced in large quantities for use in making black-and-white photographs by combining silver nitrate with potassium bromide: AgNO3(aq)+KBr(aq)AgBr(s)+KNO3(aq) Consider a reaction between 5.83m3 of 1.68MKBr reacts with 3.51m3 of 2.04MAgNO3. (Hint: 1m3=1L! ) 34. How many moles of Br - remain in solution after the AgBr precipitates? A) 7.16103molBr B) 9.70103molBr C) 2.63103molBr D) none
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


