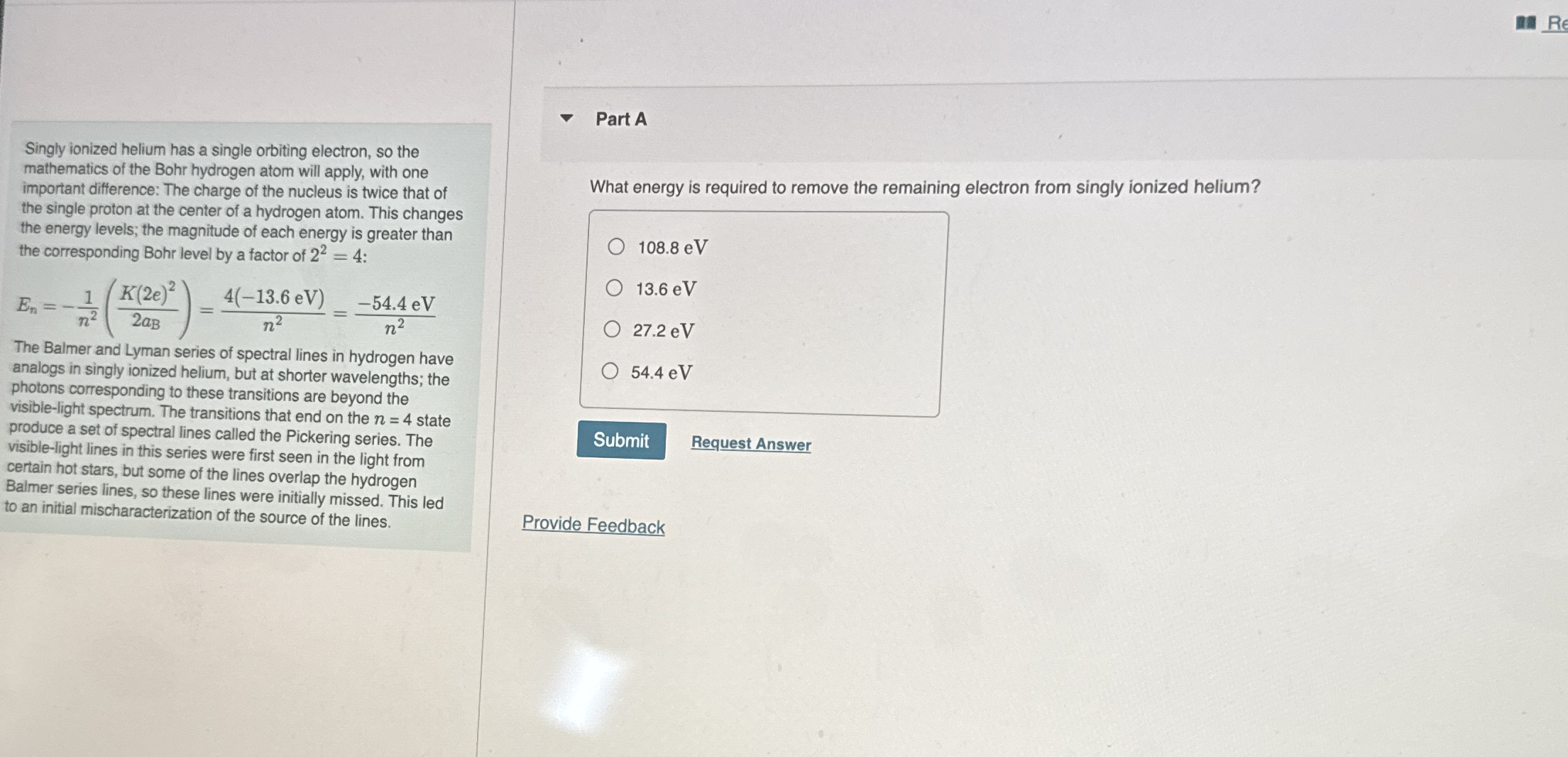
Question: Singly ionized helium has a single orbiting electron, so the mathematics of the Bohr hydrogen atom will apply, with one important difference: The charge of
Singly ionized helium has a single orbiting electron, so the mathematics of the Bohr hydrogen atom will apply, with one important difference: The charge of the nucleus is twice that of the single proton at the center of a hydrogen atom. This changes the energy levels; the magnitude of each energy is greater than the corresponding Bohr level by a factor of :
The Balmer and Lyman series of spectral lines in hydrogen have analogs in singly ionized helium, but at shorter wavelengths; the photons corresponding to these transitions are beyond the visiblelight spectrum. The transitions that end on the state produce a set of spectral lines called the Pickering series. The visiblelight lines in this series were first seen in the light from certain hot stars, but some of the lines overlap the hydrogen Balmer series lines, so these lines were initially missed. This led to an initial mischaracterization of the source of the lines.
Part A
What energy is required to remove the remaining electron from singly ionized helium?
eV
eV
eV
eV
Provide Feedback
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


