Question: Specify which of the following are oxidation-reduction reactions, and if it is, identify the oxidizing agent, the reducing agent, the substance being oxidized, and the
Specify which of the following are oxidation-reduction reactions, and if it is, identify the oxidizing agent, the reducing agent, the substance being oxidized, and the substance being reduced. If it is not, select No and leave the following boxes blank. Express your answers as a chemical formulas. Omit states-of-matter.
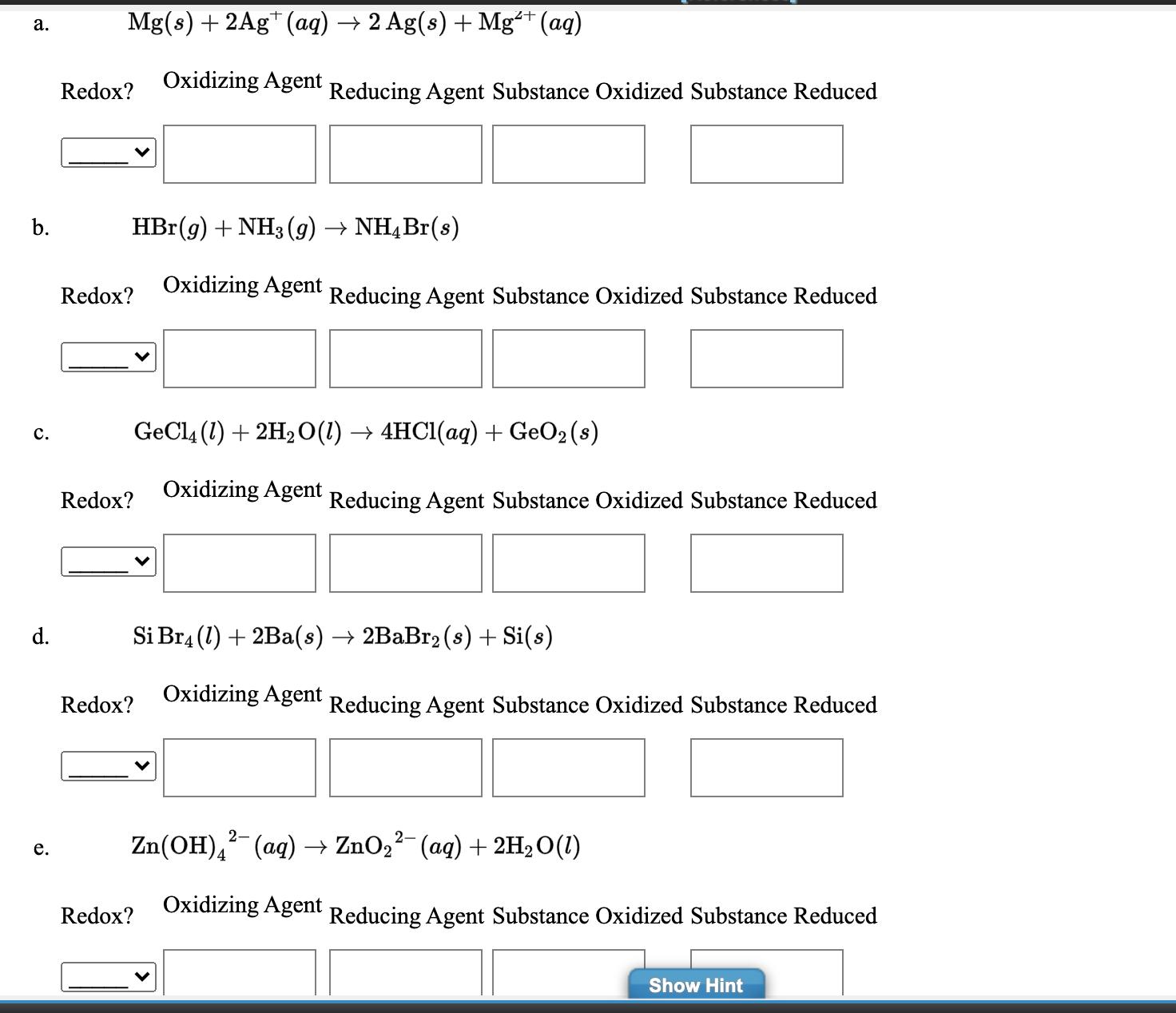
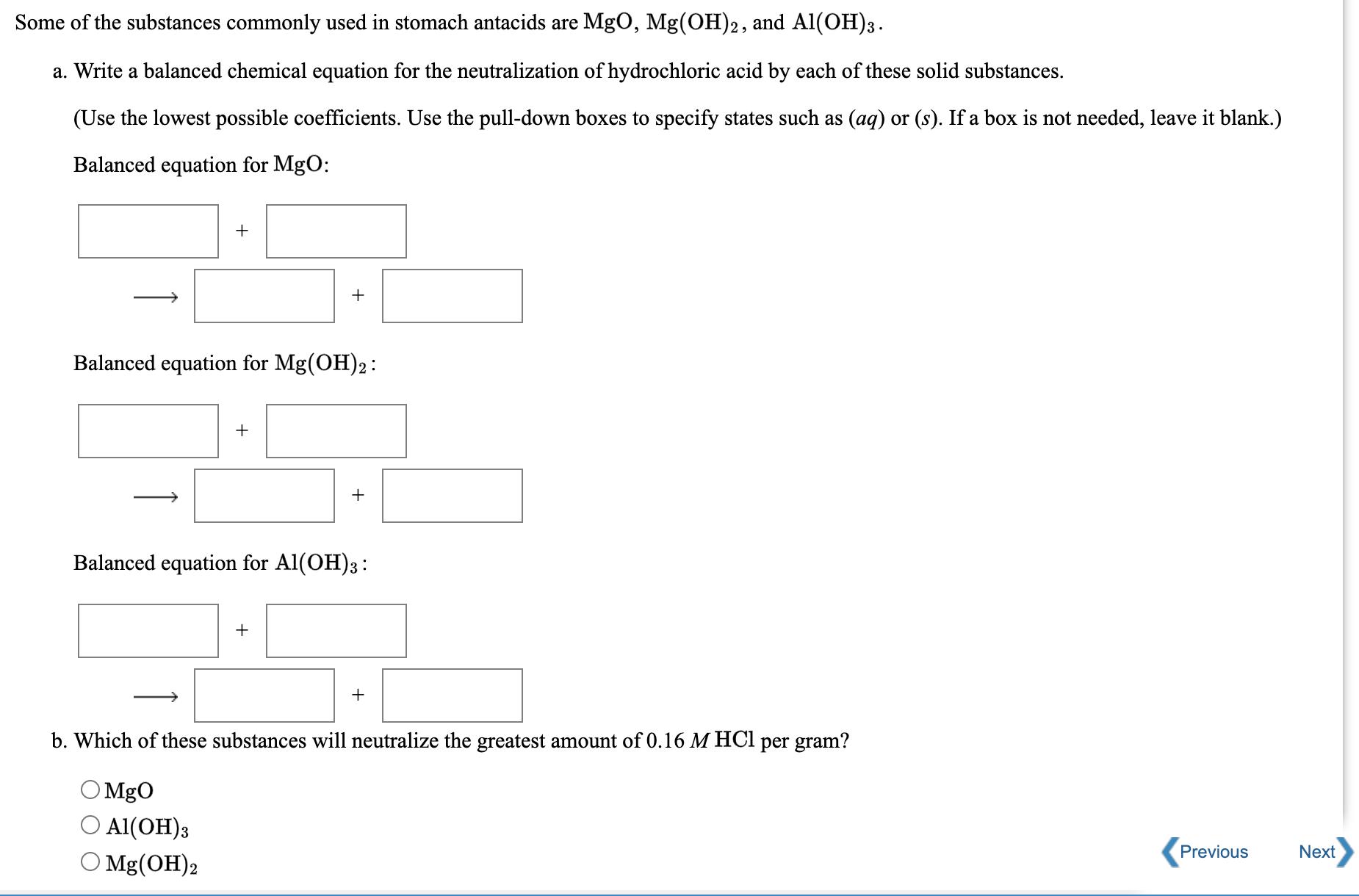
a. b. C. d. e. Mg(s) + 2Ag+ (aq) 2 Ag(s) + Mg+ (aq) Redox? Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced HBr(g) + NH3(g) NH4Br(s) Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced Redox? Redox? +7 Redox? Si Br4 (1) + 2Ba(s) 2BaBr2 (s) + Si(s) Oxidizing Agent GeCl4 (1) + 2HO(l) 4HCl(aq) + GeO (s) Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced Redox? Reducing Agent Substance Oxidized Substance Reduced 2- Zn(OH)(aq) ZnO (aq) + 2HO(1) Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced Show Hint
Step by Step Solution
3.52 Rating (165 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


