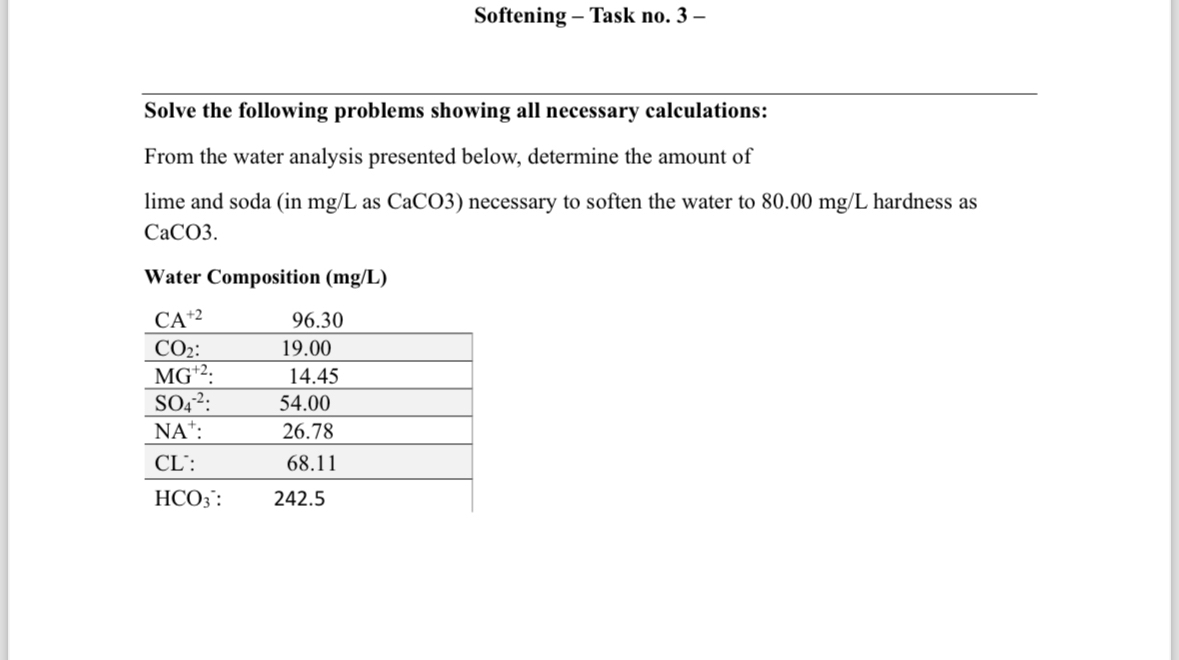
Question: Softening - Task no . 3 - Solve the following problems showing all necessary calculations: From the water analysis presented below, determine the amount of
Softening Task no
Solve the following problems showing all necessary calculations:
From the water analysis presented below, determine the amount of lime and soda in as CaCO necessary to soften the water to hardness as CaCO
Water Composition mgL
table::::::
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


