Question: Solid AgCl conducts electricity sufficiently well that the cell: A g | A g C l | C l 2 ( g ) | P
Solid AgCl conducts electricity sufficiently well that the cell:
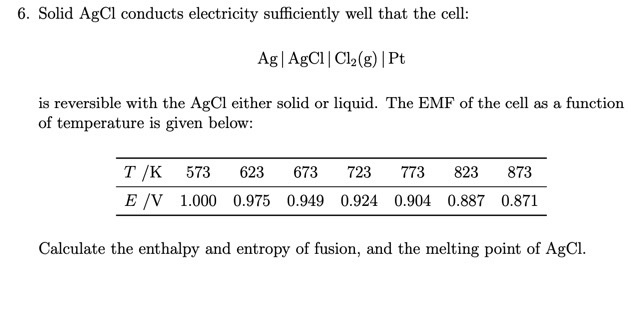
is reversible with the AgCl either solid or liquid. The EMF of the cell as a function
of temperature is given below:
Calculate the enthalpy and entropy of fusion, and the melting point of AgCl.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


