Question: Solid Unknown Molecular Formula: C3H6NOCl 1. Fill in the table below. (7 pts.) 4. Label the hydrogens in your structure with the letter of the
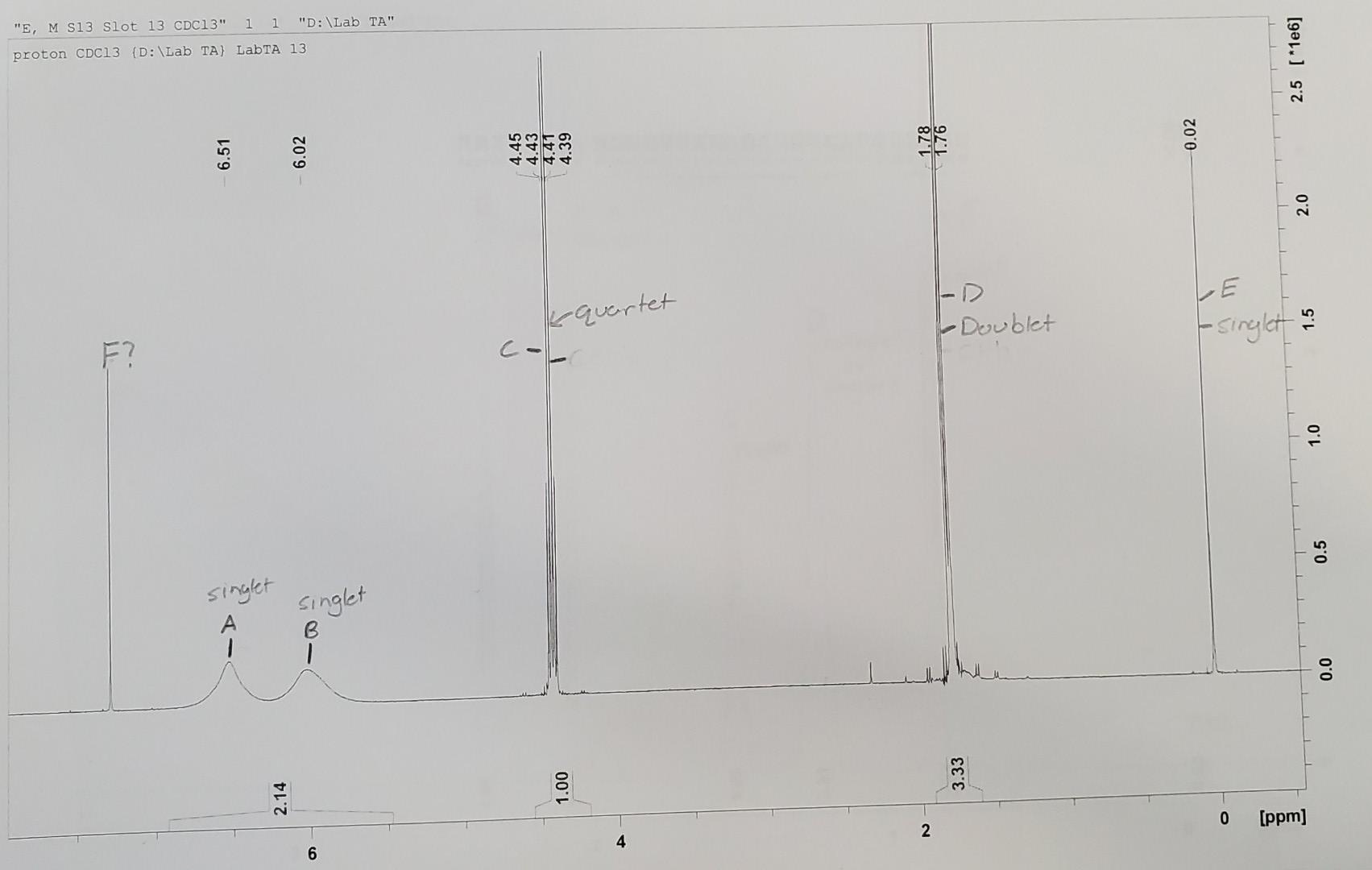
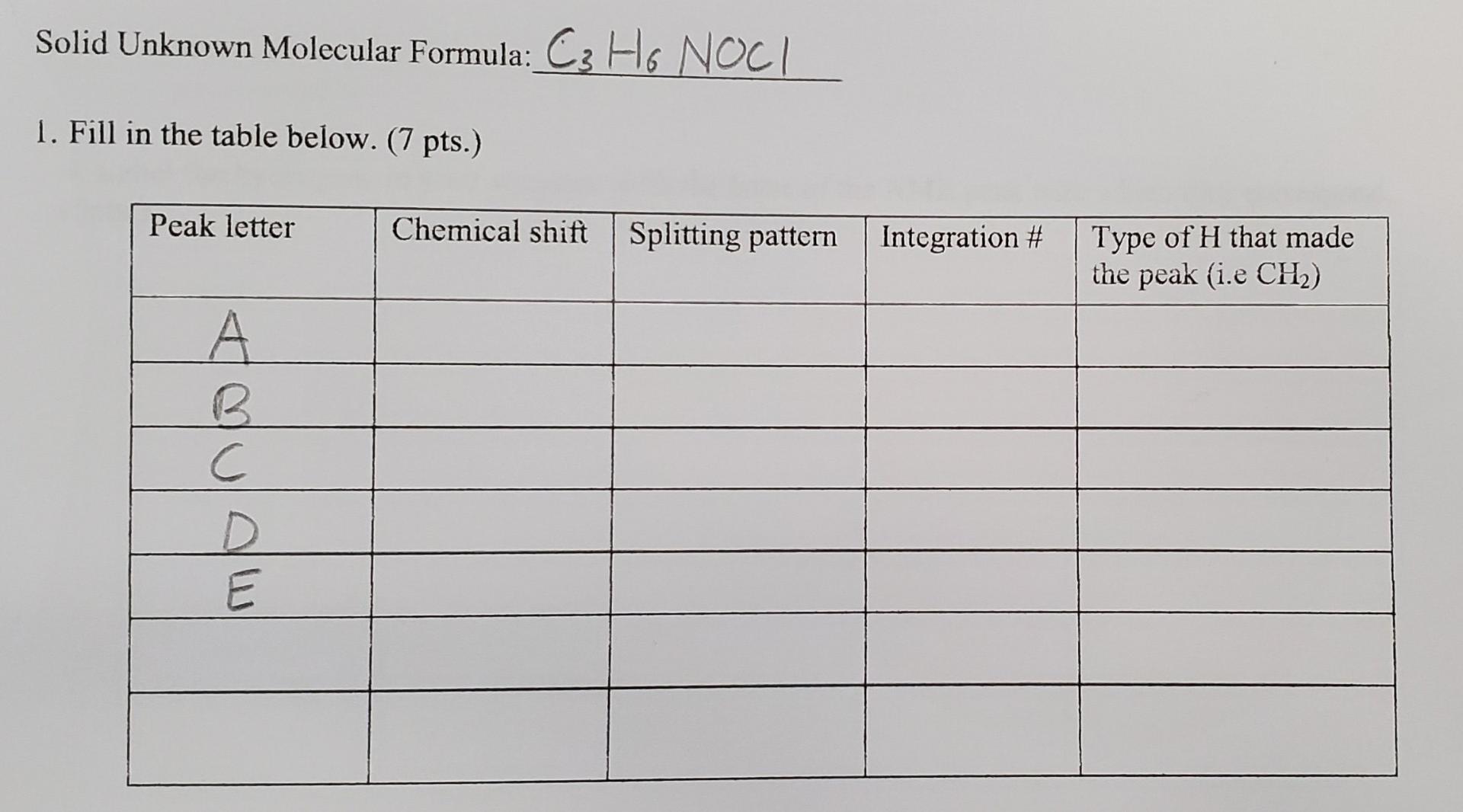

Solid Unknown Molecular Formula: C3H6NOCl 1. Fill in the table below. (7 pts.) 4. Label the hydrogens in your structure with the letter of the NMR peak with which they correspond. (5pts.) 5. Explain how you arrived at your predicted structure. For example, "The NMR contained a triplet with an integration number of 3 at 0.8. This most likely indicates the presence of a CH3 group next to a CH2 group." Be sure to mention any information that you used from your IR. (10 pts.) Solid Unknown Molecular Formula: C3H6NOCl 1. Fill in the table below. (7 pts.) 4. Label the hydrogens in your structure with the letter of the NMR peak with which they correspond. (5pts.) 5. Explain how you arrived at your predicted structure. For example, "The NMR contained a triplet with an integration number of 3 at 0.8. This most likely indicates the presence of a CH3 group next to a CH2 group." Be sure to mention any information that you used from your IR. (10 pts.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


