Question: Solubility, g solute per 100g water Part E: Solubility chart A 100.5g sample of solute B dissolved in 100g of water is decreased from 85C
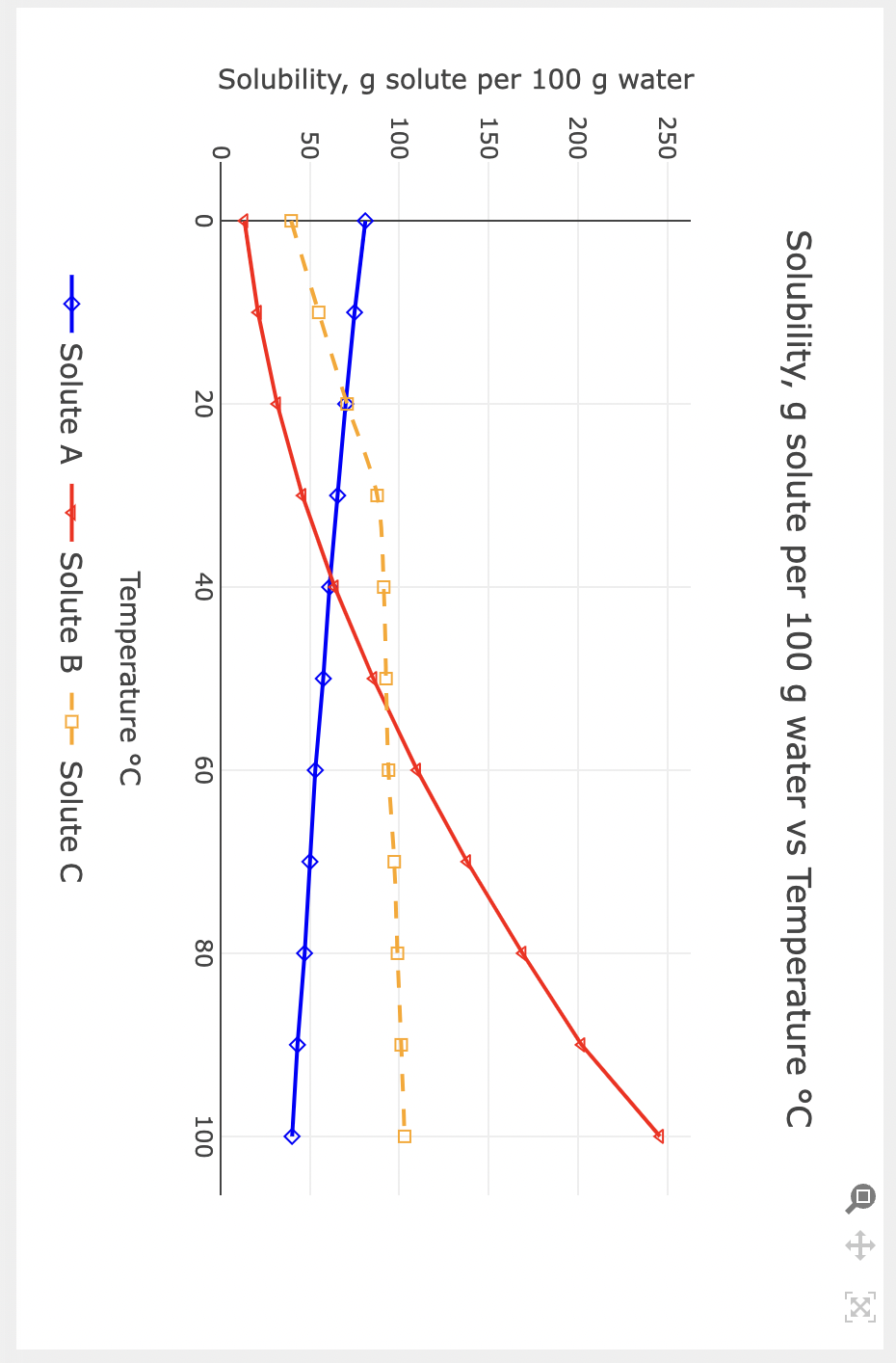
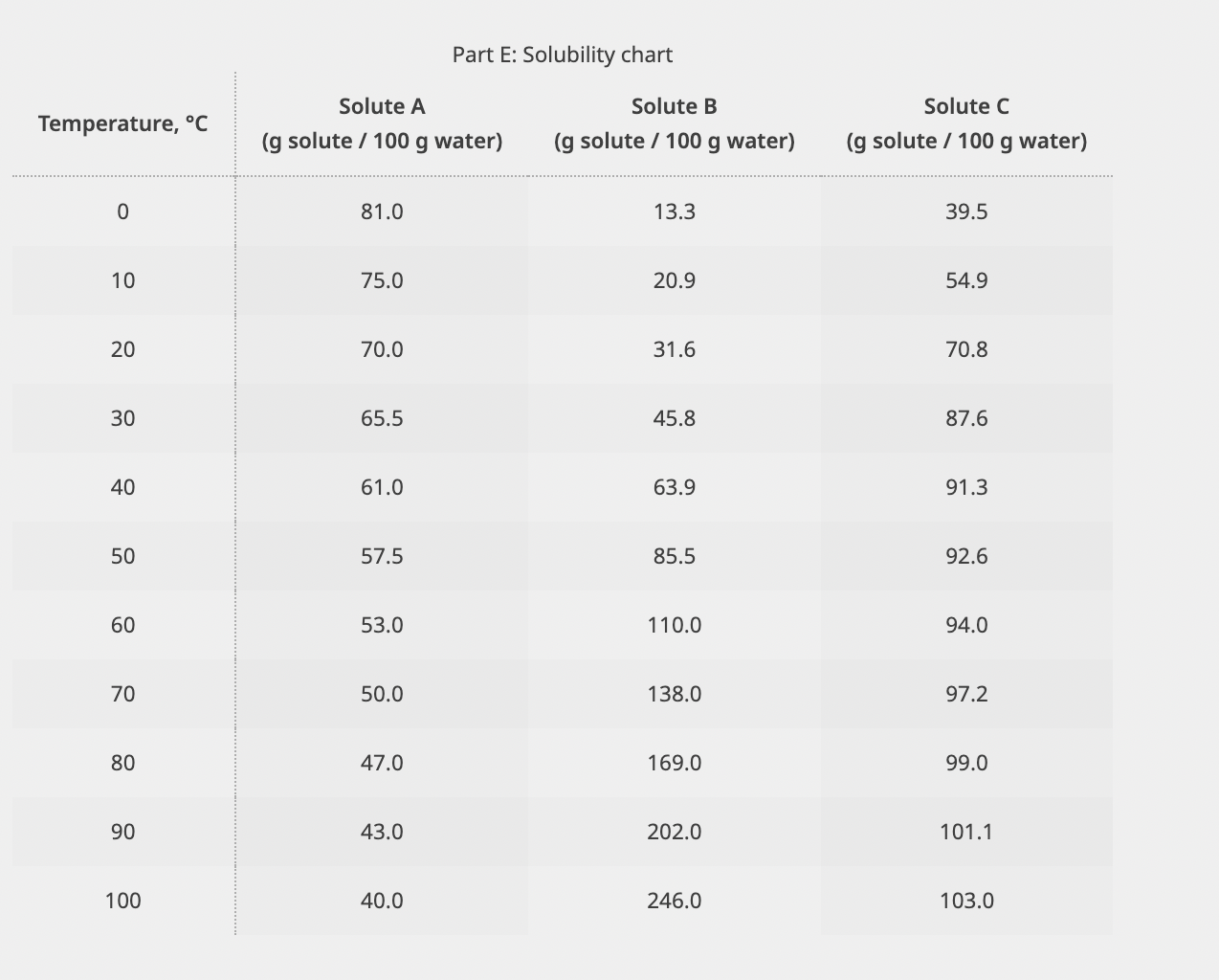
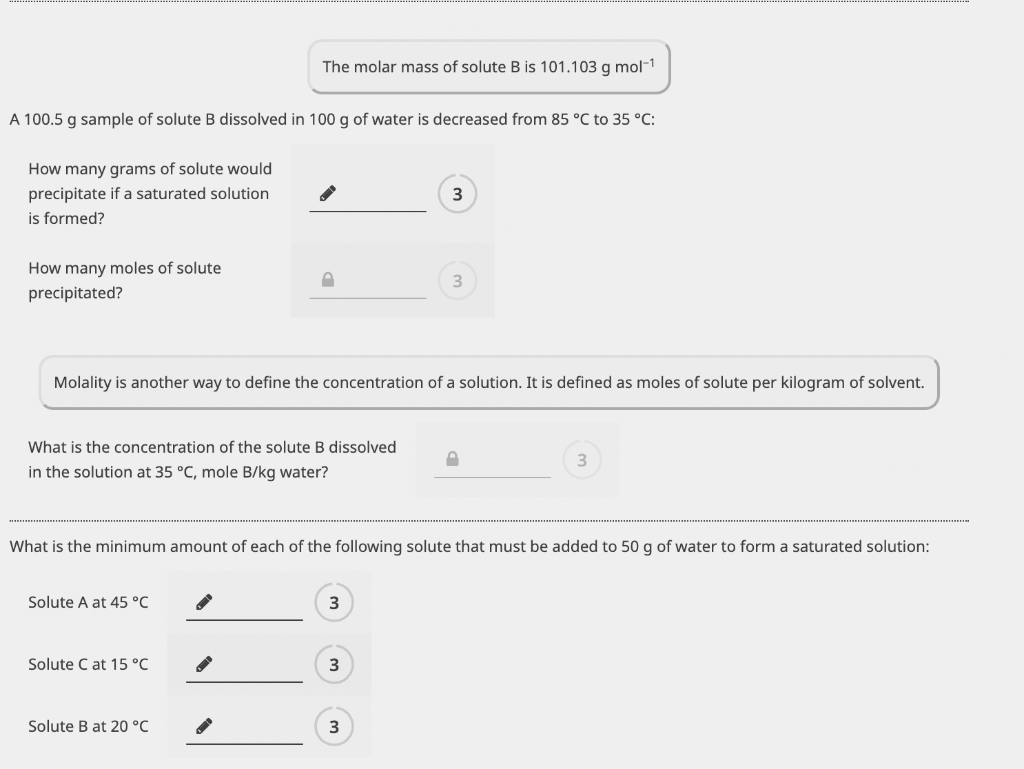
Solubility, g solute per 100g water Part E: Solubility chart A 100.5g sample of solute B dissolved in 100g of water is decreased from 85C to 35C : How many grams of solute would precipitate if a saturated solution is formed? 3 How many moles of solute precipitated? Molality is another way to define the concentration of a solution. It is defined as moles of solute per kilogram of solvent. What is the concentration of the solute B dissolved in the solution at 35C, mole B/kg water? 3 What is the minimum amount of each of the following solute that must be added to 50g of water to form a saturated solution: Solute A at 45C 3 Solute C at 15C 3 Solute B at 20C 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


