Question: solution with details please for both a and b It is proposed to produce ethanol by one of two reacuons: C2H5Cl+OHC2H5OH+ClC2H5Br+OHC2H5OH+Br Use Spartan (see Appendix
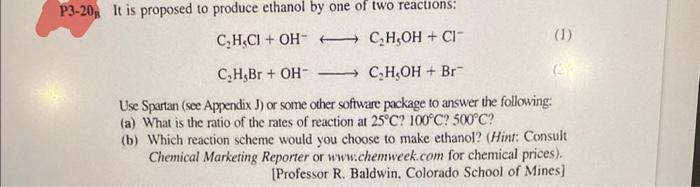
It is proposed to produce ethanol by one of two reacuons: C2H5Cl+OHC2H5OH+ClC2H5Br+OHC2H5OH+Br Use Spartan (see Appendix J) or some other software package to answer the following: (a) What is the ratio of the rates of reaction at 25C ? 100C ? 500C ? (b) Which reaction scheme would you choose to make ethanol? (Hint: Consult Chemical Marketing Reporter or www:chemweek.com for chemical prices). [Professor R. Baldwin, Colorado School of Mines] It is proposed to produce ethanol by one of two reacuons: C2H5Cl+OHC2H5OH+ClC2H5Br+OHC2H5OH+Br Use Spartan (see Appendix J) or some other software package to answer the following: (a) What is the ratio of the rates of reaction at 25C ? 100C ? 500C ? (b) Which reaction scheme would you choose to make ethanol? (Hint: Consult Chemical Marketing Reporter or www:chemweek.com for chemical prices). [Professor R. Baldwin, Colorado School of Mines]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


