Question: Solve 1 Multiple Choice 0.2 points When sodium fluoride is added to water, it dissolves and the resulting fluoride ions react with water according to
Solve
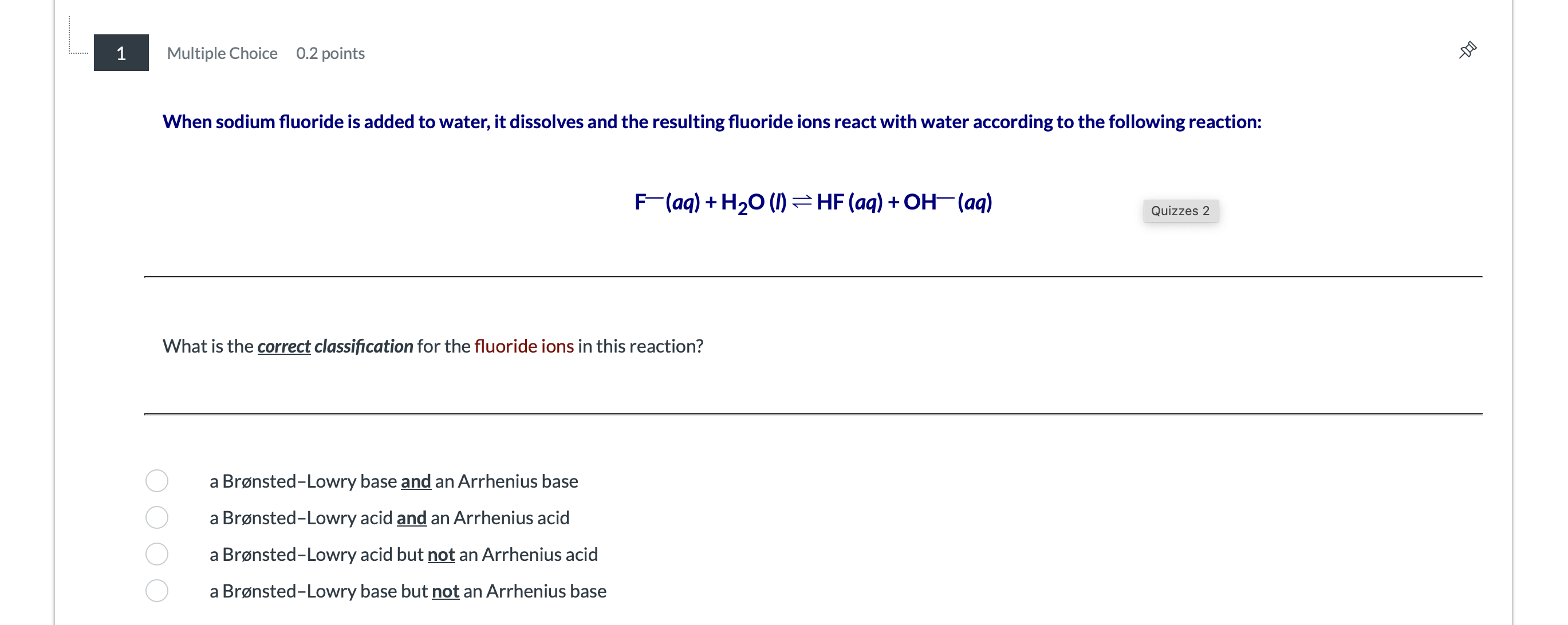 1 Multiple Choice 0.2 points When sodium fluoride is added to water, it dissolves and the resulting fluoride ions react with water according to the following reaction: F-(aq) + H20 (1) = HF (aq) + OH-(aq) Quizzes 2 What is the correct classification for the fluoride ions in this reaction? a Bronsted-Lowry base and an Arrhenius base a Bronsted-Lowry acid and an Arrhenius acid a Bronsted-Lowry acid but not an Arrhenius acid O a Bronsted-Lowry base but not an Arrhenius base
1 Multiple Choice 0.2 points When sodium fluoride is added to water, it dissolves and the resulting fluoride ions react with water according to the following reaction: F-(aq) + H20 (1) = HF (aq) + OH-(aq) Quizzes 2 What is the correct classification for the fluoride ions in this reaction? a Bronsted-Lowry base and an Arrhenius base a Bronsted-Lowry acid and an Arrhenius acid a Bronsted-Lowry acid but not an Arrhenius acid O a Bronsted-Lowry base but not an Arrhenius base
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


