Question: solve A B C D E F A total feed of 100 kmol/h having a composition of 50 mol % n-pentane and 50 mol %
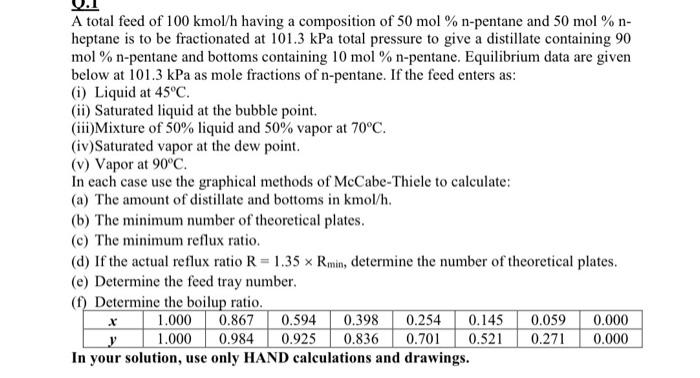
A total feed of 100 kmol/h having a composition of 50 mol % n-pentane and 50 mol % n- heptane is to be fractionated at 101.3 kPa total pressure to give a distillate containing 90 mol % n-pentane and bottoms containing 10 mol % n-pentane. Equilibrium data are given below at 101.3 kPa as mole fractions of n-pentane. If the feed enters as: (i) Liquid at 45C. (ii) Saturated liquid at the bubble point. (ii)Mixture of 50% liquid and 50% vapor at 70C. (iv)Saturated vapor at the dew point. (v) Vapor at 90C. In each case use the graphical methods of McCabe-Thiele to calculate: (a) The amount of distillate and bottoms in kmol/h. (b) The minimum number of theoretical plates. (c) The minimum reflux ratio. (d) If the actual reflux ratio R=1.35 x Rmin, determine the number of theoretical plates. (e) Determine the feed tray number (1) Determine the boilup ratio. 1.000 0.867 0.594 0.398 0.254 0.145 0.059 0.000 y 1.000 0.984 0.925 0.836 0.701 0.521 0.271 0.000 In your solution, use only HAND calculations and drawings. .x
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


