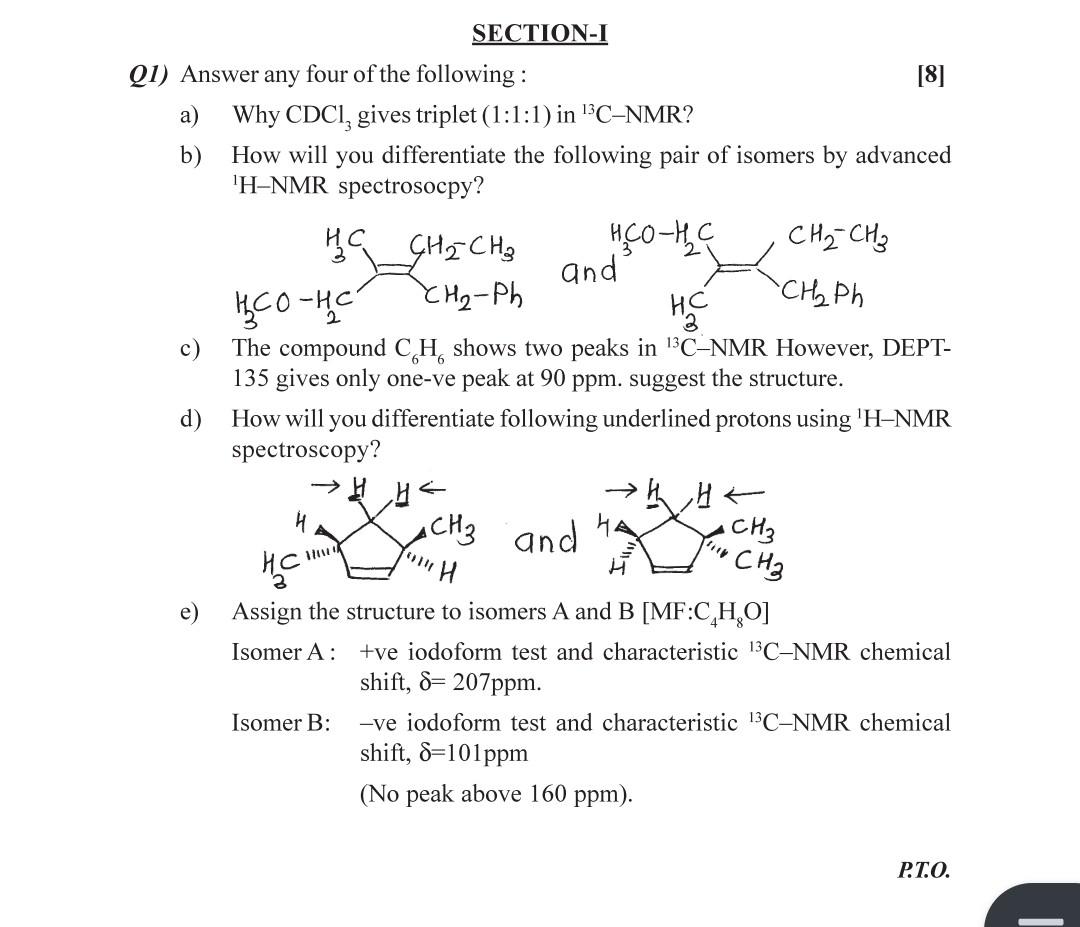
Question: solve a to e questions SECTION-I Q1) Answer any four of the following : [8] a) Why CDCl3 gives triplet (1:1:1) in 13CNMR ? b)
solve a to e questions
SECTION-I Q1) Answer any four of the following : [8] a) Why CDCl3 gives triplet (1:1:1) in 13CNMR ? b) How will you differentiate the following pair of isomers by advanced 'H-NMR spectrosocpy? c) The compound C6H6 shows two peaks in 13CNMR However, DEPT135 gives only one-ve peak at 90ppm. suggest the structure. d) How will you differentiate following underlined protons using 1HNMR spectroscopy? e) Assign the structure to isomers A and B [MF: C4H8O] Isomer A: +ve iodoform test and characteristic 13CNMR chemical shift, =207ppm. Isomer B: -ve iodoform test and characteristic 13CNMR chemical shift, =101ppm (No peak above 160ppm )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


