Question: Solve all four. 1. Write equilibrium-constant expression K, for each of the following reactions: (1 pt each) A. 2NO(g) + Br2(g) + 2NOBrg) Kp =
Solve all four.
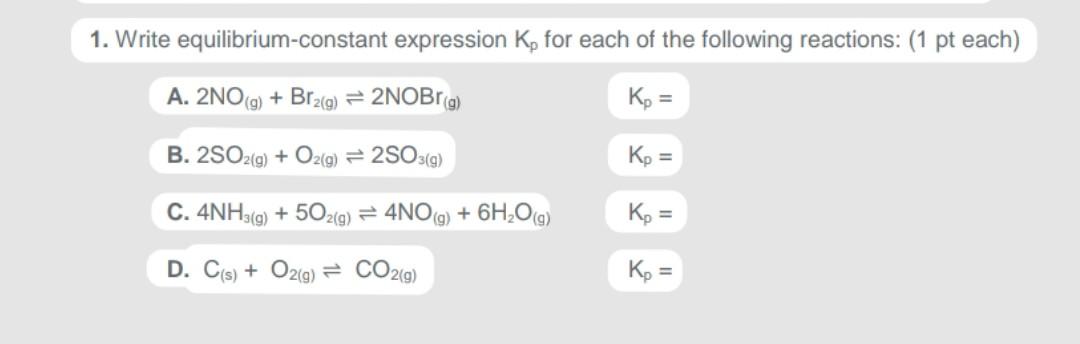
1. Write equilibrium-constant expression K, for each of the following reactions: (1 pt each) A. 2NO(g) + Br2(g) + 2NOBrg) Kp = B. 2SO2(g) + O2(g) = 2503(9) Kp = C. 4NH3(g) + 502/3) = 4NO(g) + 6H,0g) Kp = = D. C(s) + O2(g) = CO2(g) Kp = =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


