Question: solve all question 4 & 7 & 8 & 6 & 11 solve all plz 4-Fill in blanks a) In a face centred cubic (fcc)
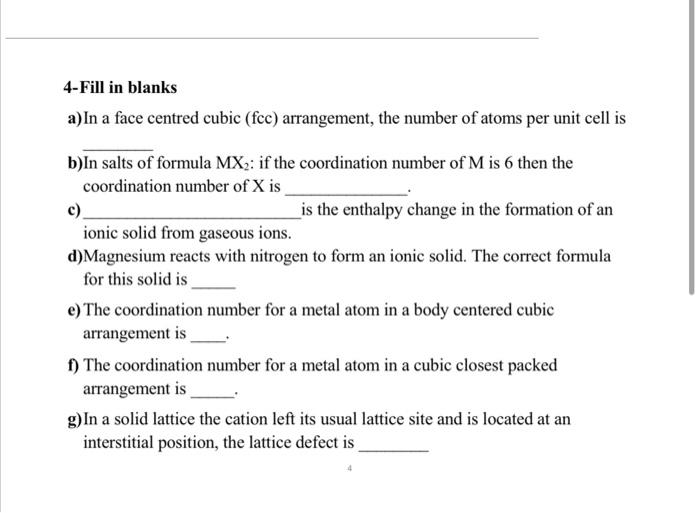
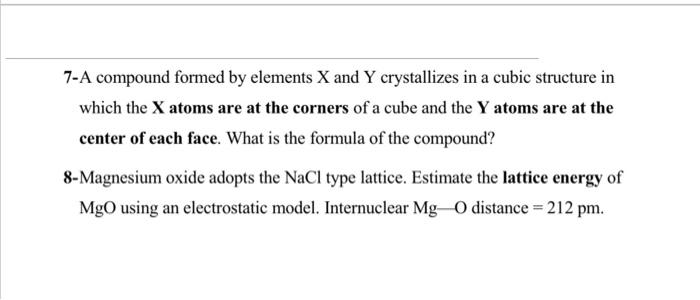

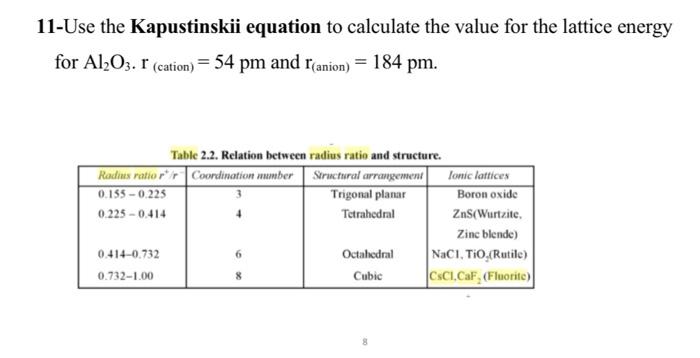
4-Fill in blanks a) In a face centred cubic (fcc) arrangement, the number of atoms per unit cell is b)In salts of formula MX2 : if the coordination number of M is 6 then the coordination number of X is c) is the enthalpy change in the formation of an ionic solid from gaseous ions. d) Magnesium reacts with nitrogen to form an ionic solid. The correct formula for this solid is e) The coordination number for a metal atom in a body centered cubic arrangement is f) The coordination number for a metal atom in a cubic closest packed arrangement is g) In a solid lattice the cation left its usual lattice site and is located at an interstitial position, the lattice defect is 7-A compound formed by elements X and Y crystallizes in a cubic structure in which the X atoms are at the corners of a cube and the Y atoms are at the center of each face. What is the formula of the compound? 8-Magnesium oxide adopts the NaCl type lattice. Estimate the lattice energy of MgO using an electrostatic model. Internuclear MgO distance =212pm. 6-If the edge-length of the unit cell of Sodium chloride (FCC structure) is 600 pm, and the ionic radius of Na+ ion is 110pm, calculate the ionic radius Cl. 11-Use the Kapustinskii equation to calculate the value for the lattice energy for Al2O3r(cation)=54pm and r(anion)=184pm. Table 2.2. Relation between radius ratio and structure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


