Question: SOLVE ALL REQUIRED DATA Exercise 4.7: Shown below are compressibility data for nitrogen: Pressure (atm) 0 10 50 100 200 300 400 600 800 Compressibility
SOLVE ALL REQUIRED DATA
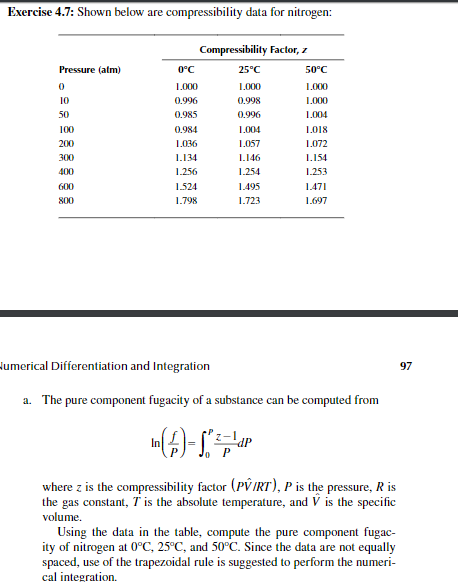
Exercise 4.7: Shown below are compressibility data for nitrogen: Pressure (atm) 0 10 50 100 200 300 400 600 800 Compressibility Factor, 0C 25C 50C 1.000 1.000 1.000 0.996 0.998 1.000 0.985 0.996 1.004 0.984 1.004 1.018 1.036 1.057 1.072 1.134 1.146 1.154 1.256 1.254 1.253 1.524 1.495 1.471 1.798 1.723 1.697 Humerical Differentiation and Integration 97 a. The pure component fugacity of a substance can be computed from In (A-S;p dP where z is the compressibility factor (PVIRT), P is the pressure, R is the gas constant, T is the absolute temperature, and V is the specific volume. Using the data in the table, compute the pure component fugac- ity of nitrogen at 0C, 25C, and 50C. Since the data are not equally spaced, use of the trapezoidal rule is suggested to perform the numeri- cal integration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


