Question: solve and show work Use the References to access important values if needed for this question. Consider the following system at equilibrium where H=87.9kJ, and
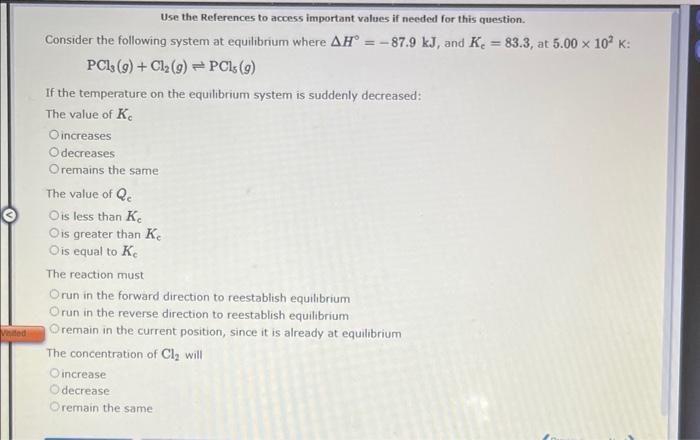
Use the References to access important values if needed for this question. Consider the following system at equilibrium where H=87.9kJ, and Ke=83.3, at 5.00102K : PCl3(g)+Cl2(g)PCl5(g) If the temperature on the equilibrium system is suddenly decreased: The value of Kc increases decreases remains the same The value of Qe is less than Kc is greater than Ke is equal to Kc The reaction must run in the forward direction to reestablish equilibrium run in the reverse direction to reestablish equilibrium remain in the current position, since it is already at equilibrium The concentration of Cl2 will increase decrease remain the same
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


