Question: Solve as soon as possible, please Solve as soon as possible, please 1. The process of steam methane reforming is summarized by two gas-phase reactions
Solve as soon as possible, please
 Solve as soon as possible, please
Solve as soon as possible, please
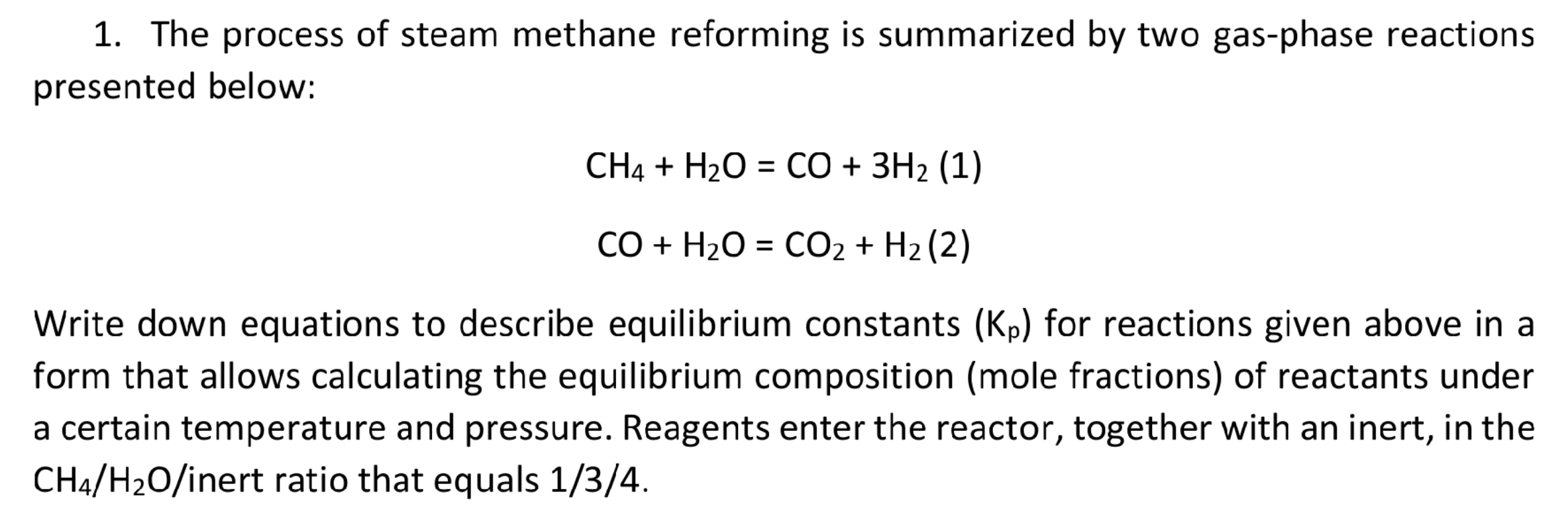
1. The process of steam methane reforming is summarized by two gas-phase reactions presented below: CH4 + H2O = CO + 3H2 (1) = CO + H2O = CO2 + H2 (2) = Write down equations to describe equilibrium constants (Kp) for reactions given above in a form that allows calculating the equilibrium composition (mole fractions) of reactants under a certain temperature and pressure. Reagents enter the reactor, together with an inert, in the CH4/H20/inert ratio that equals 1/3/4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


