Question: solve For each reaction in the table below, write the chemical formulae of any reactants that will be oxidized in the second column of the
solve
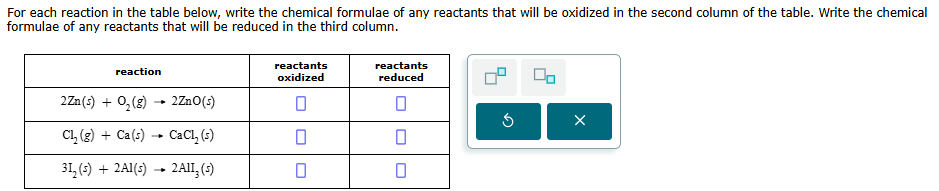 For each reaction in the table below, write the chemical formulae of any reactants that will be oxidized in the second column of the table. Write the chemical formulae of any reactants that will be reduced in the third column. reaction reactants reactants oxidized reduced 2Zn(s) + O, (8) -- 2Zno(s) 0 0 X Cl, (g) + Ca(s) -- CaCI, (s) 0 31, (5) + 2AI(5) -- 2All, (5) 0 0
For each reaction in the table below, write the chemical formulae of any reactants that will be oxidized in the second column of the table. Write the chemical formulae of any reactants that will be reduced in the third column. reaction reactants reactants oxidized reduced 2Zn(s) + O, (8) -- 2Zno(s) 0 0 X Cl, (g) + Ca(s) -- CaCI, (s) 0 31, (5) + 2AI(5) -- 2All, (5) 0 0
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


