Question: solve Hydrogen sulfide is a polyprotic acid produced naturally by decaying organic matter and has a characteristic rotten egg smell. Using the information below, calculate
solve
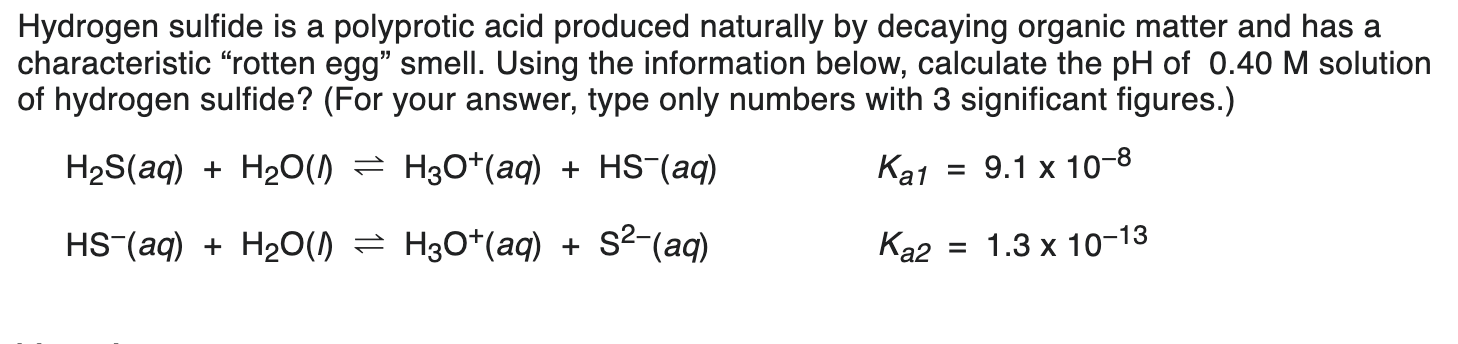 Hydrogen sulfide is a polyprotic acid produced naturally by decaying organic matter and has a characteristic "rotten egg" smell. Using the information below, calculate the pH of 0.40 M solution of hydrogen sulfide? (For your answer, type only numbers with 3 significant figures.) H2S(aq) + H20(1) - H30+(aq) + HS-(aq) Ka1 = 9.1 x 10-8 HS-(aq) + H20(1) - H30+(aq) + $2-(aq) Ka2 = 1.3 x 10-13
Hydrogen sulfide is a polyprotic acid produced naturally by decaying organic matter and has a characteristic "rotten egg" smell. Using the information below, calculate the pH of 0.40 M solution of hydrogen sulfide? (For your answer, type only numbers with 3 significant figures.) H2S(aq) + H20(1) - H30+(aq) + HS-(aq) Ka1 = 9.1 x 10-8 HS-(aq) + H20(1) - H30+(aq) + $2-(aq) Ka2 = 1.3 x 10-13 Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


