Question: Solve in Matlab Problem Description: The following data were gathered to determine the relationship between pressure and temperature of a fixed volume of 1 kg
Solve in Matlab
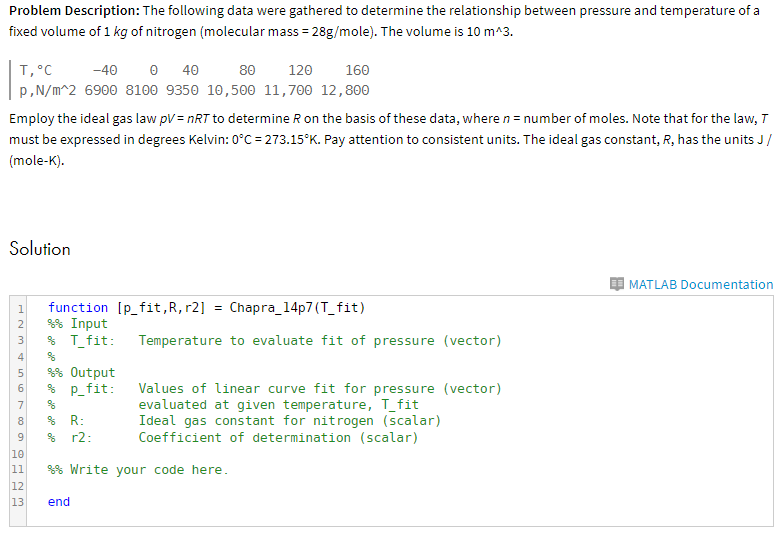
Problem Description: The following data were gathered to determine the relationship between pressure and temperature of a fixed volume of 1 kg of nitrogen (molecular mass 28g/mole). The volume is 10 mA3. 80 120 160 p,N/m 2 6900 8100 9350 10,500 11,700 12,800 Employ the ideal gas law pV= nRT to determine R on the basis of these data, where n number of moles. Note that for the law. T must be expressed in degrees Kelvin: 0C = 273.15K. Pay attention to consistent units. The ideal gas constant, R, has the units J / (mole-K). Solution MATLAB Documentation [p-fit,R, r2] = Chapra-14p7 (T-fit) function Input T-fit. 2 Temperature to evaluate fit of pressure (vector) 31 4 % Output p-fit: Values of linea r curve fit for pressure (vector) eva Luated at given temperature, Tit Ideal gas constant for nitrogen (scalar) Coefficient of determination (scalar) 6 % 8 9 % r2 : 10 111 %% write your code here 12 13 end
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


