Question: solve it and show all the steps PLEASE , I don't want any of the solutions that already exist 1. -kc dt The amount of
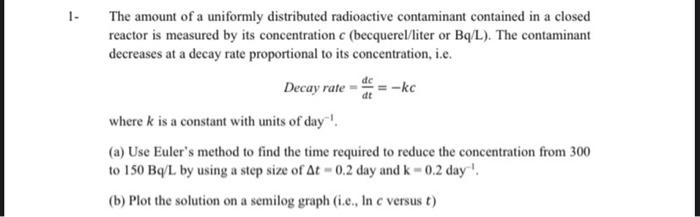
1. -kc dt The amount of a uniformly distributed radioactive contaminant contained in a closed reactor is measured by its concentration c (becquerel/liter or Bq/L). The contaminant decreases at a decay rate proportional to its concentration, i.e. Decay rate where k is a constant with units of day! (a) Use Euler's method to find the time required to reduce the concentration from 300 to 150 Bq/L by using a step size of At -0.2 day and k - 0.2 day! (b) Plot the solution on a semilog graph (i.e., In c versus t)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


