Question: solve now pls An organic flavoring agent extracted from peppermint oil. It contains C, H, and O. In one combustion analysis, 0.0100 g of the
solve now pls
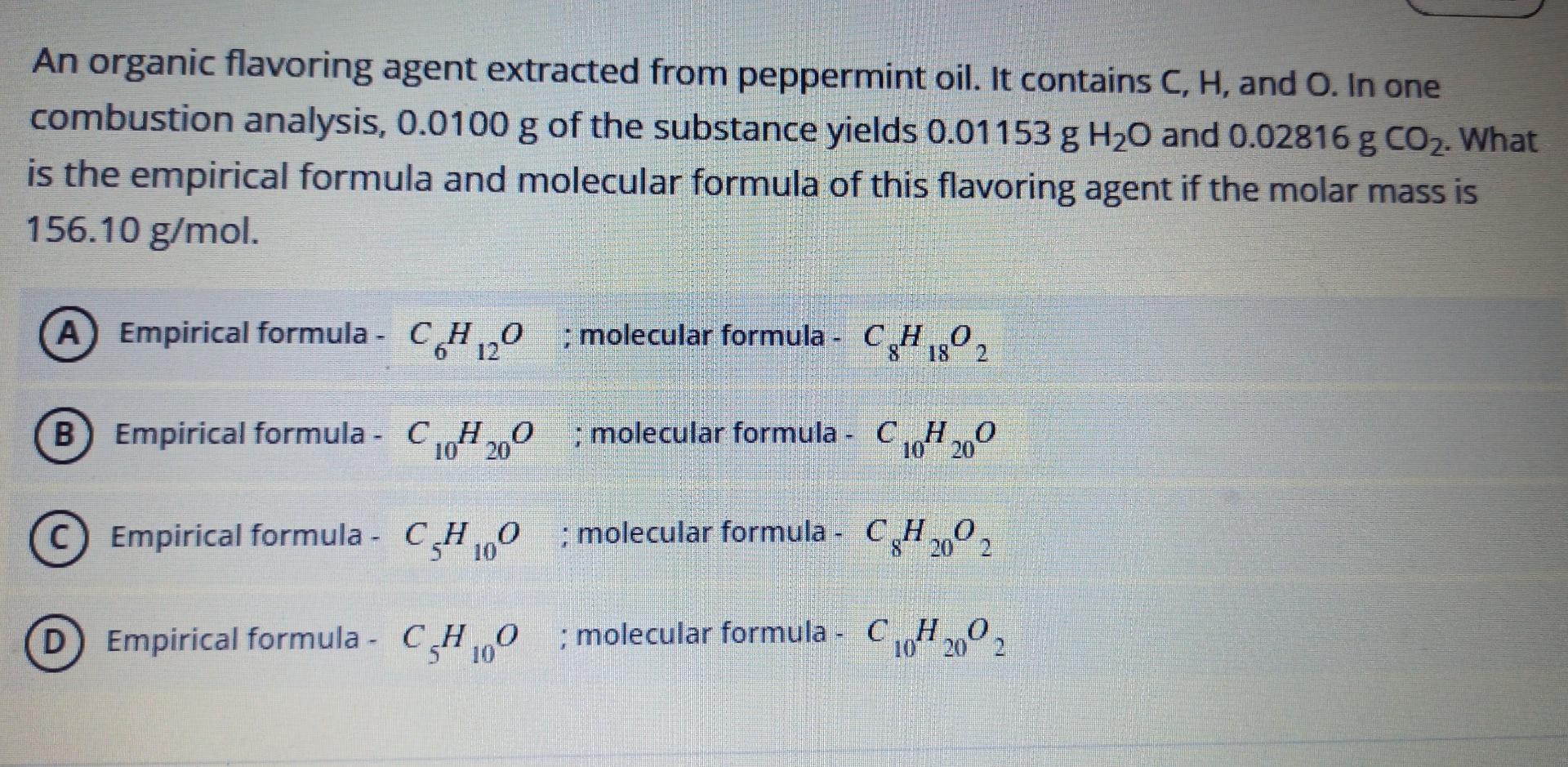
An organic flavoring agent extracted from peppermint oil. It contains C, H, and O. In one combustion analysis, 0.0100 g of the substance yields 0.01153 g H20 and 0.02816 g CO2. What is the empirical formula and molecular formula of this flavoring agent if the molar mass is 156.10 g/mol. A Empirical formula - CH20 : molecular formula - CH2 ) - 18 B Empirical formula - C H200 ; molecular formula - C10H 200 ; C 10 Empirical formula - C4,60 ; molecular formula - C H2002 : 10 D Empirical formula - CH0 : molecular formula - C H2002
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


