Question: solve Q3 and check Q4 please Initial Melting Compound Final Melting Weight (g) Temp. C Temp. C Melting Range Crude Acetanilide 3.1 108 114 Pure
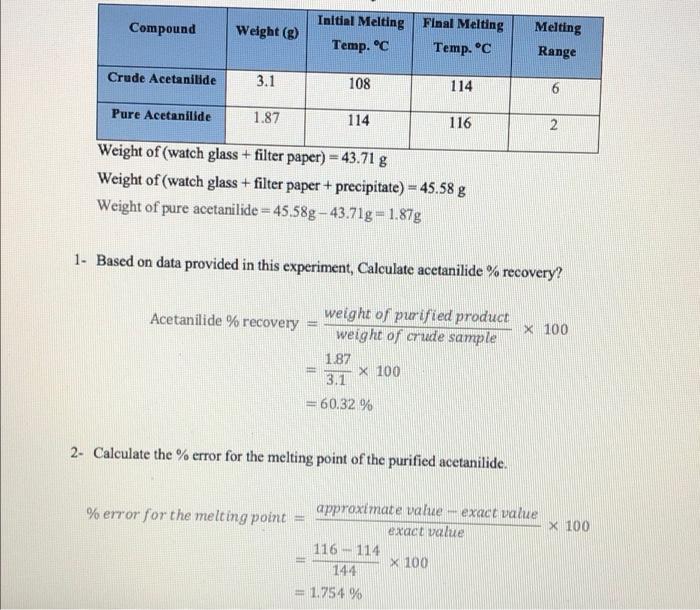

Initial Melting Compound Final Melting Weight (g) Temp. C Temp. C Melting Range Crude Acetanilide 3.1 108 114 Pure Acetanilide 1.87 114 116 2 Weight of (watch glass + filter paper) = 43.71 g Weight of (watch glass + filter paper + precipitate) - 45.58 g Weight of pure acetanilide = 45,58g 43.71g = 1.87g 1. Based on data provided in this experiment, Calculate acetanilide % recovery? Acetanilide % recovery X 100 weight of purified product weight of crude sample 1.87 X 100 3.1 - 60.32% 2- Calculate the % error for the melting point of the purified acetanilide. %error for the melting point X 100 approximate value exact value exact value 116 - 114 X 100 144 = 1.754 % 3. Based on your obtained data, indicate whether you acquired more purified acetanilide or not? Why? (Hint: Sharp M.P range means pure sample, Broad M.P range means impure sample). 4- Suppose you did not get crystals during this experiment, Answer the following: a) In which step are you? Step of cooling the solution to crystallize. b) In your opinion, What's the reason for that? Acetanilide has more solubility in hot water than in cold water. The purified solid will not recrystallize in the experiment, if we add too much hot solvent in the beginning c) How could overcome that? 1- Add a seed of original crystals 2. Scratching the side of the flask with a rod. 3. Boiling of the excess solvent to reduce the volume of the solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


