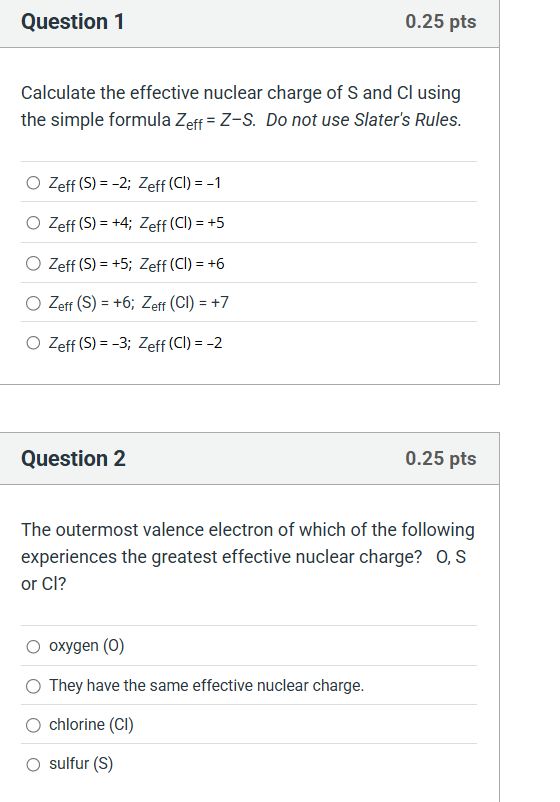
Question: solve Question 1 0.25 pts Calculate the effective nuclear charge of S and Cl using the simple formula Zeff = Z-S. Do not use Slater's
solve
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


